Abstract
Aims: The safety and efficacy of direct stenting with the Endeavor stent is unknown. The acute and 9 month outcome after direct stenting vs. predilatation was tested in a multicentre, prospective, single-arm study.
Methods and results: 300 patients were treated with the Endeavor stent for a single, previously untreated coronary artery stenosis (vessel diameter 2.25 to 3.75 mm; lesion length 14 to 27 mm). Predilatation was at discretion of the operator for lesions < 20 mm in length but mandatory for lesions > 20 mm. Angiographic follow-up at 8 months was prespecified for the first 150 consecutive patients. Out of 296 patients, 126 (42.6%) underwent direct stenting and 170 (57.4%) predilatation. Patients in the direct stenting group were younger (62.9 years vs 65.3 years, P=0.04) and lesions were predictably more proximally located and shorter (14.29 mm vs 18.16 mm, P<0.0001), with larger pre-procedural minimum lumen diameter. Success rate was 92.9% with direct stenting vs. 95.3% with predilatation (P=0.45). At 8 months, late loss was not significantly different between groups. The rate of MACE at 9 months was 10.6% with direct stenting vs. 10.2% with predilatation (P=1.00). There were no occurrences of stent thrombosis in either group.
Conclusion: Direct stenting with the Endeavor stent as deemed feasible by the operator, was safe and effective, and comparable to the results of implantation following predilatation.
Introduction
Direct stenting refers to stent positioning and deployment without prior dilatation of the stenosis. With bare metal stents, this technique has been found to be safe and feasible for the treatment of lesions in native coronary arteries1,2. The technique has also been found to be 20% to 40% less expensive than the customary predilatation procedure because it removes the cost of balloons and shortens procedural time3. The Endeavor stent combines the cobalt-alloy Driver Coronary Stent (Medtronic Vascular, Santa Rosa, CA, USA) with the antiproliferative agent zotarolimus (Abbott Laboratories, Chicago, IL, USA) that is eluted from a biomimetic phosphorylcholine polymer coating. In the pivotal Endeavor II study, a prospective, randomised, double-blind, multicentre trial of 1,197 patients, the Endeavor stent significantly reduced the rates of clinical and angiographic restenosis at 9 months as compared with the bare metal Driver stent4. It is unclear, however, whether direct stenting might affect the performance of drug eluting stents either by damaging the polymer coating or by altering the programmed drug release.
To answer this questions, 300 additional patients with stenotic lesions of native coronary arteries were added to the Endeavor stent arm of the Endeavor II study as a “continued-access registry”. The initial study protocol was altered to allow direct stenting for lesions < 20 mm in length. We report the procedural success and the acute and 9 month safety and efficacy results of direct stenting with the Endeavor system vs. deployment of the Endeavor stent following predilatation.
Methods
Patients and protocol
Fifteen sites in Germany and The Netherlands participated in this study. Patients who had single-vessel or multiple-vessel disease, with either significant stenosis or total occlusion and clinical evidence of ischaemic heart disease, or a positive function study and who were undergoing percutaneous coronary intervention for a single, previously untreated lesion in a native coronary artery were considered for enrolment. Major exclusion criteria were a left ventricular ejection fraction < 30%; significant (> 50%) stenosis proximal or distal to the target lesion; occurrence of myocardial infarction (MI) within the preceding 72 hours; contraindications or allergy to aspirin, heparin, clopidogrel, cobalt, nickel, or chromium; a history of sensitivity to contrast media; a serum creatinine level > 2.0 mg/dL (177 µmol per litre); a leukocyte count < 3,000 cells/mm3 or a platelet count < 100,000 cells/mm3 or > 700,000 cells/mm3; current participation in other investigational trials; or any coronary interventional procedure within 30 days before or after implantation of the stent system. Angiographic inclusion criteria were a reference vessel diameter of 2.25 to 3.75 mm and a lesion length of 14 to 27 mm, as estimated by the operator. Angiographic exclusion criteria included a left main or ostial target lesion or a lesion within 5 mm of the left anterior descending, left circumflex, or right coronary artery; massive calcification of the target lesion; bifurcation lesion (defined by a side branch more than 2.0 mm in diameter); and location of the target lesion at or distal to a 45º bend in the vessel. The study was conducted according to the Declaration of Helsinki, the medical ethical committees of all sites approved the study protocol, and written informed consent was obtained from every patient.
The Endeavor zotarolimus eluting stent system
The Endeavor stent received European CE Marking in August 2005. The Endeavor stent carries 10 µg of Zotarolimus per mm of stent length. Zotarolimus is a synthetic analogue of sirolimus, and its mechanism of action is similar. It binds with the intracellular protein FKBP-12 to form a trimeric complex with the mammalian target of rapamycin (MTOR), thereby blocking mTOR signal transduction, inducing cell-cycle arrest in the G1 phase and limiting cell division5-7. The polymer phosphorylcholine coating is a synthetic copy of the outer membrane of red blood cells and thereby has a high biovascular compatibility. In animal studies, phosphorylcholine-coated stents have demonstrated significantly less platelet adhesion compared with uncoated stents8. Delivery of ABT-578 by means of phosphorylcholine-coated stainless steel stents into the arteries of juvenile domestic pigs was found to significantly reduce neointimal formation at 28 days compared with controls9. The Endeavor I First-in-Man prospective trial of 100 consecutive patients with symptomatic ischaemic heart disease due to de novo obstructive lesions of native coronary arteries, found that conventional deployment of the Endeavor stent was safe and feasible and provided sustainable clinical outcomes to 12 months with in-stent late loss of 0.33 and 0.61 mm, at 4 and 12 months respectively10. The incidence of major adverse cardiac events (MACE) was 1% at 30 days and 2% at 4 and 12 months.
Stent implantation
Endeavor stents were implanted according to a standardised procedure. Before catheterisation, patients received 300 mg of aspirin and a 300 mg loading dose of clopidogrel. During the procedure, unfractionated heparin was administered to maintain activated clotting time > 250 sec or between 200 and 250 sec if a glycoprotein IIb/IIIa inhibitor was given. After stenting, patients were prescribed 75 mg of aspirin daily indefinitely and 75 mg of clopidogrel for 3 months.
Predilatation was at the operator’s discretion for lesions that were readily accessible and < 20 mm in length. If lesions were > 20 mm in length and either moderately tortuous or calcified, predilatation was mandatory. Stents were available in lengths of 18, 24, and 30 mm. In the event of edge dissection or incomplete coverage, additional stents could be implanted, at the operator’s discretion, up to a maximum of 48 mm. Postdilatation using short balloons was performed at the operator’s discretion. Clinical follow-up was scheduled at 30 days, 6 months, 9 months, and yearly thereafter for 5 years. In addition, the first 150 enrolled patients were scheduled to undergo angiographic follow-up at 8 months.
Data management
The computerised database was maintained by the Harvard Clinical Research Institute (Boston, MA, USA) to which the principal investigators had unrestricted access. All major adverse cardiac events (MACE) were reviewed and adjudicated by an independent committee.
Endpoints and definitions
The primary endpoint was the 9-month MACE rate, defined as a composite of death, recurrent Q-wave or non-Q-wave MI, emergent cardiac bypass surgery, or target lesion revascularisation (TLR). TLR was defined as repeat revascularisation for ischaemia owing to stenosis > 50% of the lumen diameter anywhere within the stent or within the 5 mm borders proximal or distal to the stent. Myocardial infarction was defined either as the development of pathologic Q-waves in at least two contiguous leads with or without elevated cardiac enzymes or, in the absence of pathologic Q-waves, an elevation in creatinine kinase levels to greater than twice the upper limit of normal in the presence of an elevated creatinine kinase MB level.
Secondary endpoints were device-deployment success, defined as attainment of < 50% residual stenosis of the target lesion by means of the Endeavor stent; lesion success, defined as attainment of < 50% residual stenosis of the target lesion by any percutaneous method; procedure success, defined as attainment of < 50% residual stenosis of the target lesion without a major adverse cardiac event in 30 days; angiographic late loss, defined as the difference between minimum lumen diameter (MLD) immediately after the procedure and MLD at 8 months; and binary restenosis, defined as stenosis of > 50% of the lumen diameter of the treated lesion. All angiographic measurements were reported separately for the vessel section within the stent (“in stent”), for the vessel portions extending 5 mm from the proximal and distal edges of the stent, and for the entire segment (“in segment”).
Stent thrombosis was defined as an acute coronary syndrome with angiographic documentation of vessel occlusion or thrombus within or adjacent to the stented segment. In the absence of angiographic documentation, stent thrombosis could be confirmed by acute MI in the distribution of the treated vessel or death from cardiac causes within 30 days.
Statistical analysis
Categorical variables were compared by means of the likelihood-ratio chi-square test or Fisher’s exact test. Continuous variables are presented as means±SD or medians with interquartile ranges and were compared with the use of the Student’s t-test or the Wilcoxon two-sample test. All P values are two-sided.
Results
Baseline characteristics and procedural results
The Endeavor zotarolimus eluting stent system was deployed in 296 patients of which 126 (42.6%) received the Endeavor stent by direct stenting (one patient had two lesions treated by direct stenting) and 170 (57.4%) received the Endeavor stent after predilatation.
The baseline and lesion characteristics of the two groups were well matched except that patients who underwent direct stenting were younger (62.87 years vs 65.27 years, P=0.04) (Table 1).
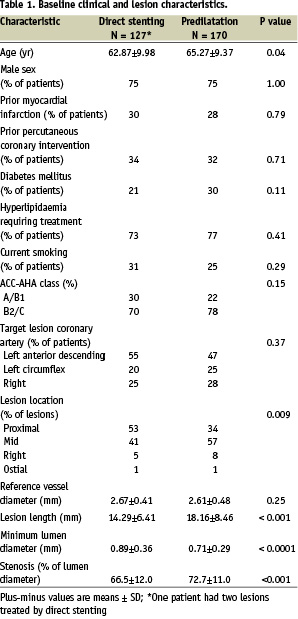
Because balloon predilatation was mandatory for lesions > 20 mm, it was expected that patients in the predilatation group would have more complex lesions than patients in the direct stenting group. The lesions in the predilatation group were significantly longer (18.16 mm vs 14.29 mm, P<0.001) and more distally located (only 34.1% of the lesions were proximal in the predilatation group vs. 53.5% in the direct stenting group). The initial MLD was smaller (0.71 mm vs 0.89 mm, P<0.0001) and the initial percent diameter stenosis was greater (72.7% versus 66.5%, P<0.001) in the predilatation group. Because predilatation was mandatory for lesions > 20 mm, it was expected that patients in the predilatation group would receive longer stents than patients in the direct stenting group (stent length 27.3 vs 21.9 mm, P<0.001). The baseline difference in angiographic metrics, reflecting the initial difference in the complexity of lesions between the treatment groups, predictably remained after stent deployment (Table 2).
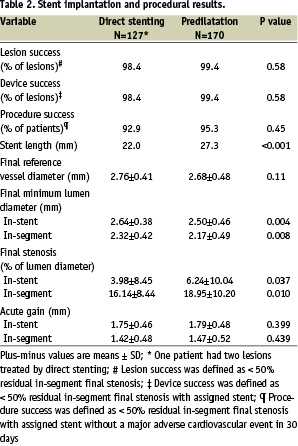
In contrast, there was no significant difference between groups in acute gain, defined as the immediate dimensional change in MLD between baseline and post-procedure (Table 2). The in-segment acute gain was 1.42 mm for direct stenting vs. 1.47 mm for predilatation (P=0.44). The acute lesion and device-deployment success rates approached 100% in the two groups (Table 2). The acute procedural success rate was 92.9% after direct stenting vs. 95.3% after predilatation. Stent placement using the chosen approach was successful in all patients and there were no crossovers from direct stenting to balloon predilatation.
Clinical outcome
At 9 months, 123 patients (96.8%) in the direct stenting group and 166 patients (97.6%) in the predilatation group were available for analysis (Table 3).
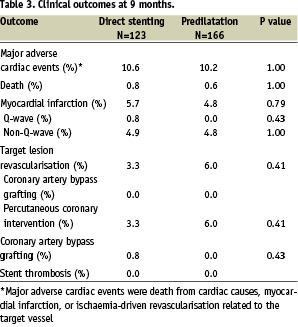
MACE rate with direct stenting was 10.6% vs. 10.2% with predilatation (P=1.00). TLR rate was 45% less in the direct stenting than in the predilatation group, but this difference did not reach statistical significance (P=0.41). One patient in the direct stenting group experienced emergency coronary artery bypass grafting. There was no occurrence of acute or late stent thrombosis in any patient (0/296).
Angiographic results
Follow-up angiography was completed at 8 months for 62 patients (49.2%) in the direct stenting and 85 patients (50.0%) in the predilatation group (Table 4).
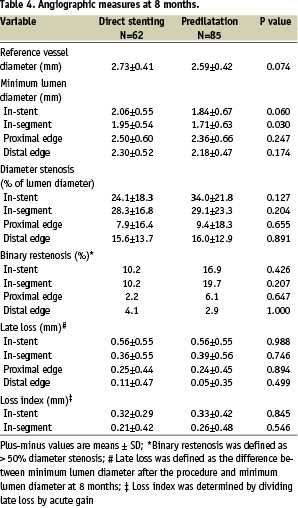
In-stent and in-segment late loss was comparable between groups. The in-segment binary restenosis rate was 48.2% less in the direct stenting vs. the predilatation group (10.2% vs 19.7%, P=0.21).
Discussion
Direct stenting has been compared with conventional stenting after balloon predilatation in a number of prospective, randomised trials1,2. With direct stenting, the procedural outcome seems to be superior to that with predilatation because of a reduced incidence of dissections at stent edges11. However in 4 randomised trials, no difference in restenosis rate or clinical outcome was found between either approaches12-15. A modest reduction in procedural and equipment costs, together with the reduced exposure time to fluoroscopy, has been confirmed in the randomised trials1,2.
The current observational study is the only prospective trial to date of direct stenting with a drug-eluting stent. Direct stenting was permitted at the operator’s discretion for lesions that were readily accessible and <20 mm in length. Measurements of acute postprocedural gain and late loss at 8 months showed no significant differences between the direct stenting and the predilatation groups. There was no significant difference in clinical endpoints at 9 months. The analysis shows that for less complex lesions chosen at the operator’s discretion, direct stenting with the Endeavor stent is feasible and equally efficacious as balloon-facilitated stent delivery.
Direct stenting with other drug-eluting stents
The safety and efficacy of direct stenting with a sirolimus eluting stent (Cypher, Cordis Corporation, Miami, FL, USA) and with a paclitaxel eluting stent (Taxus, Boston Scientific, Natick, MA, USA) have been evaluated in two post hoc subgroup analyses16,17.
Direct stenting was left at the operator’s discretion in the European and Canadian Sirolimus Eluting Stent trials (C-SIRIUS18 and E-SIRIUS19) and 57 of the 225 patients (25.3%) received the sirolimus eluting stent by direct stenting16. The directly stented and the predilatated patients in these trials had similar baseline characteristics, except that patients in the direct stenting group had a lower prevalence of moderate to severe lesion calcification (5% vs 19%, P=0.017) and a lower baseline diameter stenosis (61.6% vs 68.1%, P<0.001). At 1 year, the clinical follow-up showed reduced TLR (1.8% vs. 5.4%, P=0.46) and MACE rates (5.3% vs. 8.9%, P=0.57) favouring direct stenting, although the differences did not reach statistical significance18.
Direct stenting was left at the operator’s discretion in the TAXUS II randomised controlled trial22. Of the 536 patients receiving a paclitaxel eluting stent or a bare metal stent in this study, direct stenting was performed in 49 patients (9.1%), 23 in the paclitaxel eluting-stent group and 26 in the control group17. At 6 months, there was a nonsignificant trend for lower MACE and reduced in-stent restenosis rates in patients receiving the paclitaxel eluting stent by direct stenting vs. predilatation group.
At this point, a major limitation of the present prospective study with Endeavor and the post hoc analyses of direct stenting with the sirolimus and paclitaxel eluting stents is the lack of randomisation.
Conclusion
In this prospective, single-arm, multicentre study of patients with previously untreated coronary lesions, the implantation of the Endeavor zotarolimus eluting stent by means of direct stenting was as safe and effective as the implantation of the Endeavor stent with prior balloon dilatation.
Appendix
This study was supported by Medtronic Vascular, Santa Rosa, CA.The following investigators and institutions participated in the prospective, open-label, multicentre study of the Endeavor stent: H-P. Schultheiss, Universitätsklinikum Benjamin Franklin, Berlin; E. Grube, Krankenhaus & Herzzentrum, Siegburg; K-H. Kuck, Hamburg Krankenhaus Sankt Georg, Hamburg; M. Suttorp, St. Antonius Ziekenhuis, Nieuwegein; H. Heuer, Medizinische Klinik St. Johannes, Dortmund; T. Münzel, Universitätsklinikum, Hamburg-Eppendorf; W. Rutsch, Universitatsklinikum Charité, Berlin; G. Laarman, Onze Lieve Vrouwe Gasthuis, Amsterdam; E. Hauptmann, Krankenhaus der Barmherzigen Brüder, Trier; P. Sick, Universitat Leipzig Herzzentrum, Leipzig; J. Bonnier, Catharina Ziekenhuis, Eindhoven; S. Silber, Private Praxis Muenchen; A. Zeiher, Klinikum der J-W Goethe, Frankfurt; C. Hamm, Kerckhoff Klinik, Bad Nauheim; B. Hennen, Universitätskliniken des Saarlandes, Homburg.

