Abstract
Aims: To provide insight on the patients with long lesions that received multiple, overlapping stents by reporting clinical outcomes, together with a detailed angiographic and intravascular ultrasound analysis of the overlap zone.
Methods and results: TAXUS VI is a prospective, multicentre, double-blind, trial, specifically designed to assess outcomes of paclitaxel-eluting stents in longer lesions by randomising 446 patients (1:1) between a drug-eluting TAXUS™ Express2 Moderate Release (MR) and an uncoated Express2 control stent. Multiple overlapping stents were implanted in 124 patients (27.8%) and are the subject of this report. Clinical, angiographic and IVUS outcomes at nine months were compared in the overlap group for patients receiving the TAXUS™ Express2 MR stent and the uncoated Express2 stent. In the overlap group, mean lesion length was 25.1 mm with a mean stent length of 43.6 mm. At nine months, TVR was reduced by 94% from 25.0% to 1.6% in the TAXUS patients compared with control (p=<0.0001); TLR was reduced by 93% from 23.3% to 1.6% (p=0.0002). Binary restenosis in the stented area was reduced by 89% from 45.5% in the control patients to 4.8% in the TAXUS patients (p<0.0001). There was a trend towards a higher 30-day myocardial infarction rate in the TAXUS group (7.9% vs. 1.6%; p=0.21), but by nine months, MACE was significantly reduced by 62.0%, from 25.0% to 9.5% in favour of the TAXUS patients (p=0.0305). Late acquired incomplete apposition on IVUS was infrequent in either group and was not located in the overlap zone, nor was the finding associated with late MACE. No stent thrombosis occurred in either group.
Conclusions: The use of multiple overlapping TAXUS™ MR stents appears safe and effective in the treatment of long complex coronary artery lesions.
Introduction
Drug-eluting stents offer dramatic improvements in clinical and angiographic indices when single stents are used for treatment of simple de novo lesions. There are limited and inconsistent published data establishing the risk-benefit profile in patients treated with multiple overlapping drug-eluting stents1-4.
Implantation of multiple stents presents technical challenges in delivery and alignment to insure adequate stent overlap and lesion coverage. Deliberate stent overlap is a recognised option for the treatment of in-stent restenosis for both bare metal and drug-eluting stents, including the TAXUS stent5. Theoretical concerns related to the use of overlapping drug-eluting stents include the potential toxic effect of ‘double’ doses of a drug on the vascular wall which may result in positive remodelling, aneurysm formation, and possible stent thrombosis, as well as aggravated restenosis as a consequence of increased implantation injury and delayed healing; potential adverse reactions to a polymer delivery system in terms of inflammation and thrombogenic effects may also increase with overlapping stents6,7. Furthermore, the differing dose-response curves and the pharmacokinetics of the available drugs may have variable effects on the local vascular biology, so that it may not be possible to extrapolate the results from one drug-eluting stent to another.
TAXUS VI is a prospective, randomised, double-blind study that demonstrated superior results for the TAXUS polymer-based paclitaxel moderate release stent compared with bare metal stents for the treatment of long lesions8. The purpose of this report is to provide insight on the patients with long lesions that received multiple, overlapping stents by reporting clinical outcomes, together with a detailed angiographic and intravascular ultrasound analysis of the overlap zone.
Methods
Paclitaxel-eluting stent system
In the TAXUS VI trial, the TAXUS™ stent consists of a balloon expandable Express2 stent with a triblock copolymer coating containing paclitaxel (1 µg/mm2 of paclitaxel [loaded drug/stent surface area]). The moderate release (MR) polymer formulation used in this study releases approximately three-fold higher paclitaxel release by in vitro preclinical assessment compared with the commercially available slow release formulation. An uncoated Express2 stent (hereafter referred to as the control stent) served as the control in the study.
Patient selection
Patient entry criteria have been described previously8. Patients eligible for percutaneous coronary intervention and acceptable candidates for coronary artery bypass grafting provided written informed consent. Angiographic inclusion criteria included a de novo target lesion(s) located within a single native coronary vessel with a reference vessel diameter of ≥2.5 mm to ≤3.75 mm. In the TAXUS VI study, target lesions could be composed of multiple lesions defined as diameter stenosis ≥50%; the region with the lesion(s) had to be completely coverable by up to two study stents (maximum allowable lesion length of ≥18 mm and ≤40 mm covered by a stent length of up to 48 mm).
Study procedure
The study was approved by the local medical ethics committee in each of the investigation sites. Patients who met the study entry criteria were randomised to receive treatment with the TAXUS™ stent or the control stent using an Interactive Voice Response System (IVRS). Patients were pre-treated with aspirin 75 mgs and clopidogrel 300 mgs (at least two hours prior to intra-coronary stents implantation according to the instructions-for-use); balloon pre-dilatation prior to stent insertion was mandated. Angiographic images were recorded according to the protocol of the angiographic core laboratory. A pre-discharge electrocardiogram was obtained and cardiac enzyme estimations were taken 12-24 hours after stent placement. Aspirin ≥75 mgs daily and clopidogrel 75 mgs daily were continued for a minimum of six months after the procedure. Glycoprotein IIb/IIIa inhibitors were used at the discretion of the physician.
Angiographic analysis
Coronary angiography was performed at baseline, immediately post-PCI, and at the time of 9-month angiographic follow-up. Qualitative and quantitative angiographic analysis was performed by an independent core laboratory blinded to both treatment assignment and clinical outcomes, using a validated computer-assisted edge detection algorithm and standardised definitions as previously described9-12. Lesion length was defined as the axial extent of the lesion that contained a shoulder-to-shoulder lumen reduction by ≥20% or more. Restenosis patterns were qualitatively assessed using the Mehran classification system13. Coronary aneurysms were defined as a maximum lumen diameter within the treatment zone that was 1.2 or more times larger than the average reference diameter of the vessel.
Overlap stent zones were identified by the relative position of the stent balloon markers and background radiographic markers, such as side-branches, before and after the procedure and at follow-up. The length of the overlap zone and the mean, minimal, and maximum lumen dimensions were specifically measured in the overlap zones.
IVUS imaging and analysis
IVUS imaging was performed at experienced pre-specified clinical sites with contemporary IVUS equipment. After intracoronary administration of 0.1-0.2 mg nitroglycerin and using a motorised pullback (0.5 mm/s), images were continuously recorded throughout the stent and at least 5 mm distal and proximal to the stent. Images were recorded onto SVHS videotape or digitally onto CD or MO disc and analysed at an independent core laboratory at the Washington Hospital Center (Washington, D.C., USA).
Using computerised planimetry, the reference segment external elastic membrane (EEM), stent, and lumen were measured every millimetre within the stent and up to 5 mm proximal and distal to each stent edge. Volumes were calculated using Simpson’s rule and baseline and follow-up images were aligned using the stent edge. Volumes were calculated only if the IVUS-determined stent length matched the known stent length and the vascular volume was calculated if the EEM interface was visualised every millimetre throughout the stent. Incomplete stent apposition was defined as a separation of at least one stent strut from the intimal surface of the arterial wall; incomplete apposition at 9-months was considered ‘late-acquired’ if it was not present post-implantation.
The stent overlap section was identified by confirming double-struts in the overlap section identified by measurements from the stent edge. Neointima volumes in the overlap sections were calculated separate from neointima volumes in the non-overlap sections in these patients.
Clinical endpoints
All patients were contacted by a trained study coordinator at 30 days, six months, and nine months after randomisation to determine the occurrence of clinical endpoints. The primary endpoint of TAXUS-VI and of the present analysis was the occurrence of ischaemia-driven target vessel revascularisation (TVR). Additional endpoints included death (cardiac or non-cardiac), myocardial infarction, and ischaemia-driven target lesion revascularisation (TLR). TVR and TLR were considered to be ischaemia-driven if the stenosis of the target vessel (as determined by the angiographic core laboratory) was at least 50% of the luminal diameter, with either resting electrocardiographic changes or a functional study indicating ischaemia in the distribution of the target vessel, or if there was stenosis of at least 70% in conjunction with recurrent symptoms alone. Myocardial infarction was categorised as Q-wave, and non-Q wave; Q-wave infarction was defined as the development of new pathological Q-waves in two or more leads lasting 0.4 seconds or more with post procedure CK-MB levels elevated above normal; non-Q wave infarction was defined as the presence of post-procedure CK levels >2.0 times normal with positive CK-MB.
An independent committee, blinded to treatment assignment, adjudicated events against the definitions. A data monitoring committee periodically reviewed blinded safety data.
Data management and statistical analysis
Site monitoring, data management and analysis were undertaken by an independent organisation (PPD Development, D-90402 Nurenberg, Germany); following unblinding, the investigators had unrestricted access to the data. We compared clinical outcomes, stratified by treatment assignment and whether patients received multiple, overlapping stents. Continuous variables are described as mean ± standard deviation and were compared by unpaired t-tests. Categorical variables are presented as frequencies and were compared using chi-square tests.
All analyses were performed on an intention to treat basis; patients who were assigned to undergo angiographic follow-up were thus considered to be in the angiographic follow-up group regardless of whether angiographic follow-up was actually performed. All analyses were performed using SAS version 8.2 (Cary, NC), and a p-value <0.05 was considered statistically significant for all comparisons.
Role of the funding source
The study sponsor, Boston Scientific Corporation, Natick, Massachusetts, USA, supported the design of the study, the collection and analysis of the data, and participated in the writing of the report.
Results
Baseline characteristics
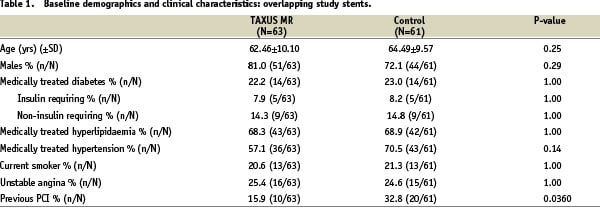
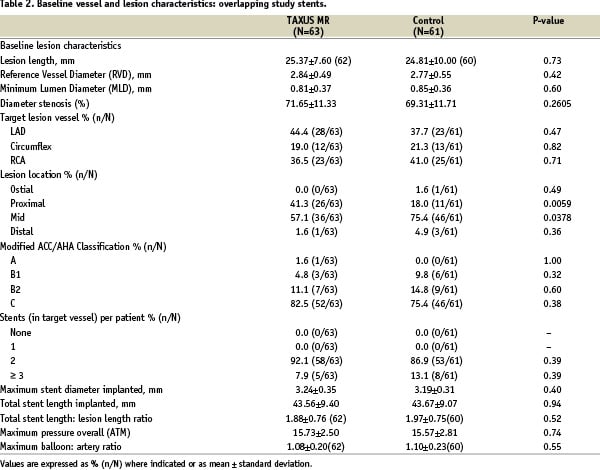
Of the 446 patients originally randomised in TAXUS VI, multiple stents were implanted in 166 patients (37.2%), 83 patients in the TAXUS group, and 83 patients in the control group. Overlapping study stents were used in 124 patients (27.8% of the total), 63 patients in the TAXUS group and 61 patients in the control group (Figure 1), which form the basis for this study. Within the overlapping study stent group, patients receiving TAXUS and control stents were well-matched for baseline demographics, vessel and lesion characteristics, except for previous PCI, which was more common in the control group by chance (Tables 1 & 2).
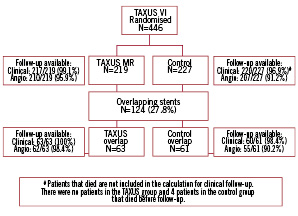
Figure 1. Patient flow.
Clinical outcomes for the overlapping study stent group
In patients treated with multiple overlapping study stents, TVR was reduced from 25.0% (15/60) in the control group to 1.6% (1/63) in the TAXUS group (relative reduction 94%, p<0.0001) at nine months; target lesion revascularisation (TLR) in patients treated with overlapping study stents was reduced from 23.3% (14/60) in the control group to 1.6% (1/63) in the TAXUS group (relative reduction 93%, p=0.0002) (Figure 2).
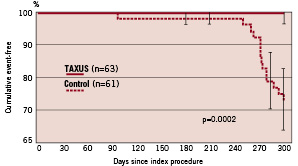
Figure 2. Freedom from target lesion revascularisation (TLR): Overlapping study stents.
For both in-hospital and 30 days, the overall MACE was 7.9% in the TAXUS MR group compared with 1.6% in the control group (p=.21), all were procedure related.
At nine-month follow-up, no cardiac deaths occurred in either group. Overall MACE (death, acute myocardial infarction, target vessel revascularisation) in the overlap group at nine months was significantly less in the TAXUS patients at 9.5%, compared with the control patients at 25.0% (p=0.0305) as detailed in Table 3.
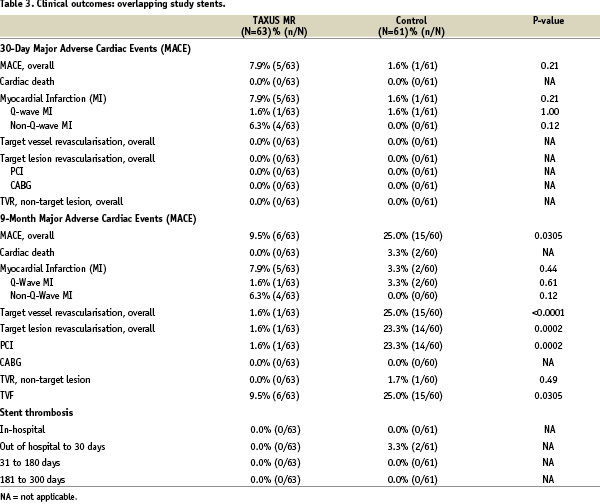
There was no stent thrombosis reported in either the TAXUS or control overlap stent groups out to nine months.
Angiographic outcomes for the overlapping study stent group
Follow-up nine month angiography was available in 62/63 patients (98.4%) in the TAXUS group and 55/61 (90.2%) of the control group (Table 4).
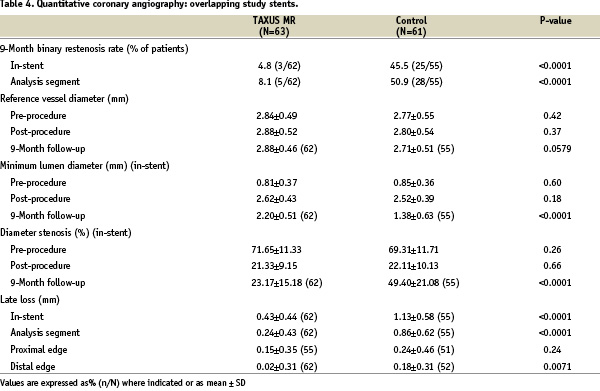
In-stent binary restenosis was reduced from 45.5% in the control patients to 4.8% in the TAXUS patients (relative reduction 89%, p<0.0001). In-segment binary restenosis (including the stented area plus the proximal and distal 5 mm edge), was reduced from 50.9% in the control patients to 8.1% in the TAXUS patients (relative reduction 84%, p<0.0001).
Late lumen loss (reduction in minimum lumen diameter from post-procedure to follow-up) in the overlapping study stent group was reduced from 1.13±0.58 mm in the control group to 0.43±0.44 mm in the TAXUS group (p<0.0001) in stent, and from 0.86±0.62 mm in the control group to 0.24±0.43 mm in the TAXUS group in the analysis segment (p<0.0001). The length of in-stent restenosis in the TAXUS group was shorter than in the control group in patients treated with a single stent (11.9±7.3 mm and 16.4±8.1 mm respectively, p=0.09), and with overlapping study stents (8.1±3.9 mm and 18.7±9.8 mm respectively, p=0.08); although a trend, these differences were not significant. There was a significant shift from diffuse to focal restenosis in the TAXUS patients treated with overlapping study stents; diffuse restenosis occurred in 12/55 (21.8%) in the control patients, and in 1/63 (1.6%) of the TAXUS patients (p=0.0006).
Angiographic analysis of the overlap segment
A detailed QCA analysis of the overlap region is shown in Table 5.
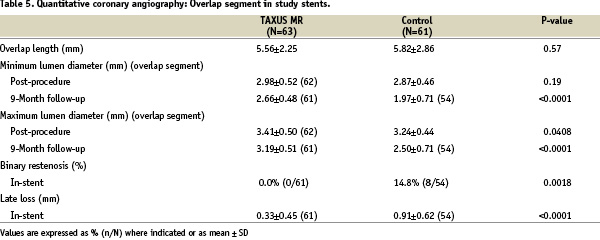
Overlap length was similar in both groups (TAXUS 5.56±2.25, control 5.82±2.86 mm, p=0.57). There was a relative reduction of in-stent late loss in the overlap region of 64% from 0.91±0.62 in the control group to 0.33±0.45 in the TAXUS group (p<0.0001); in-stent binary restenosis in the overlap region was reduced from 14.8% to 0% (p=0.0018). At nine month angiographic follow-up, late acquired aneurysms (defined as vessel distension ≥1.2 times reference vessel diameter present at follow-up but not post procedure) were observed in one TAXUS overlap patient (1.6%) and in no control overlap patients (p=n.s.).
Intravascular ultrasound analysis
An IVUS substudy was completed in 170 patients of which 130 had paired post-procedure and nine month studies suitable for detailed core laboratory analysis, 67 patients in the TAXUS group and 63 in the control group. Of these, 15/67 TAXUS patients and 20/63 control patients with overlapping study stents had paired analysable IVUS images. In patients with overlapping study stents, percentage net volume obstruction assessed by IVUS was reduced from 30.83±14.70% in the control patients to 7.91±7.64% (relative reduction 74%, p<0.0001). Late acquired incomplete apposition occurred in four overlap patients (2/22 TAXUS, 9.1%; 2/21 control, 9.5%, p=1.00). In none of these patients was the incomplete apposition located in the overlap zone, nor was incomplete apposition associated with MACE at nine-month follow-up.
Discussion
The early trials in the TAXUS program demonstrated that treatment of simple lesions with polymer-based paclitaxel-eluting stents was more effective at reducing the need for target lesion revascularisation compared with bare metal stents14-17. The later TAXUS V and TAXUS VI trials extend these results to the more complex patient and lesion subsets treated in the ‘real world’, including those with longer lesions, smaller reference vessel diameters, and in-stent restenosis3,8,18. Given the limitations in the presently available maximum drug-eluting stent lengths of 32 mm for TAXUS and 33 mm for CYPHER stents, multiple overlapping stents are inevitably used for treatment of very long lesions, despite the limited data relating to this approach. Operators favour an overlap strategy because of the practical difficulties with abutting stents, coupled with concern over gaps or geographic miss. The biologic effects of multiple drug dosing will also impact on the vascular biology of patients treated with either the ‘crush’ or the ‘culotte’ bifurcation techniques.
Theoretical concerns related to the use of overlapping drug-eluting stents include the potential toxic effect of increased doses of a drug on the vascular endothelium19 which may result in positive remodelling and aneurysm formation, and possible late stent thrombosis as a consequence of incomplete re-endothelialisation. Potential adverse reactions to a polymer delivery system in terms of inflammation and thrombogenic effects7 may also increase with overlapping stents.
The TAXUS MR (moderate release) investigational preparation used in this trial releases 33 µgm of paclitaxel per 3.0 x 24 mm stent over 30 days (data from a pre-clinical animal model), approximately three times the dose released from the commercially available TAXUS SR (slow release) preparation. Overlapping moderate release TAXUS stents establish a clinical safety margin for the slow release preparation used in daily clinical practice.
We report the first detailed analyses of multiple, overlapping stents, from the pre-specified sub-group of the long lesion subset of TAXUS VI. Despite the treatment of very long lesions (mean 25.1 mm) and higher stent to lesion ratios (mean 1.94), and the practicalities associated with the more complex overlap procedure, there were improvements in efficacy parameters compared with control stents (93% reduction in TLR and 89% reduction in binary restenosis), without excessive edge stenosis. If in-stent restenosis occurred in the TAXUS overlap patients, there was a significant shift to the focal category (mean length of 8.1 mm) compared with the control group where the pattern commonly involved diffuse and proliferative lesions (mean length 18.7 mm). Angiographic analysis of the overlap region (double stent layer with mean length ~5.7 mm) compared to single stent regions showed comparable late loss (0.33 mm), absence of restenosis and preserved patterns of TAXUS superiority to control for minimum lumen.
By 30 days post-procedure, MACE were seen more frequently in the TAXUS group, although the excess was not significant (TAXUS 5/63, 7.9%; control 1/61, 1.6%; p=0.21) which may have reflected the small sample size. The main component of MACE in the TAXUS group was peri-procedural non-Q wave myocardial infarction (TAXUS 4/63, 6.3%; control 0/61, 0%; p=0.12). After nine months, there was no additional non-Q wave infarction in the TAXUS group, and MACE in the control group was significantly higher due to a preponderance of TLR (TAXUS nine month MACE 6/63, 9.5%; control MACE 15/61, 24.6%; p=0.0316). An increase in early MACE was also a feature of the drug-eluting limb of the TAXUS V trial3 which used the TAXUS SR (slow release) stent. Periprocedural myocardial infarction (mainly non Q-wave) was significantly higher in the TAXUS group (TAXUS 8.3%, control 3.3%; p=0.047), which was attributed to side-branch compromise and reduced side-branch flow; IVUS data were not reported. This observation did not however translate into an increased incidence of death, stent thrombosis, or other late sequelae.
At nine month follow-up in the present study, no episodes of stent thrombosis were recorded in either the TAXUS (0/61; 0%) or the control (0/63; 0%) groups, despite the fact that dual antiplatelet therapy with aspirin and clopidogrel was reduced to aspirin alone after six months.
Deliberately overlapping two TAXUS MR stents will result in the overlap segment receiving a higher dose of paclitaxel19. Detailed QCA assessment of the overlap region revealed that this dose resulted in a low rate of late loss of 0.33 mm, with not a single episode of in-stent binary restenosis in the 61 patients treated with overlapping TAXUS stents, a finding which may be related to the higher paclitaxel dose. Furthermore, there was no evidence of any increase in vessel size at follow-up in the overlap region in the TAXUS group from the analysis of either the minimum lumen diameter (2.98±0.52 mm vs. 2.66±0.48 mm), or the maximum lumen diameter (3.41±0.50 mm vs. 3.19±0.51 mm) post-procedure and at nine-month angiographic follow-up respectively, both of which might have occurred had there been evidence of drug toxicity. Late incomplete apposition was identified by intravascular ultrasound in four overlap patients (three TAXUS vs. one control), but did not occur in the overlap zone, nor was it associated with MACE.
Similar favourable results have also been reported in a small IVUS series of eight patients treated with CYPHER stents1. Tsagalou et al reported a series of 66 patients with left anterior descending disease treated with overlapping TAXUS (27/66, 41%) or CYPHER (39/66, 59%) stents2. A high incidence (16.6%) of peri-procedural non-Q wave myocardial infarction (TAXUS 6/27, 22.2%; CYPHER 5/39’ 12.8%), was attributed to the complex nature of the intervention. In this study, patients were not randomised, nor was there a bare metal control group. Clinical follow-up was available in all patients with a TVR rate of 15% (10/66); six month angiography was undertaken in 52 patients (79%) which demonstrated a binary restenosis rate of 19.6%. A recent meta-analysis of five CYPHER trials has also reported favourable results with overlapping drug eluting compared with bare metal stents4. 337 patients with overlapping stents (337 CYPHER, 238 bare metal) demonstrated greater late lumen loss and angiographic restenosis compared with single stents, regardless of stent type, without evidence of an increase in peri-procedural myocardial infarction in the CYPHER group. Clinical restenosis was increased in the bare metal overlap group, but not in the CYPHER group compared with single stents. Differences in stent architecture, polymer, drug release characteristics and dosing effects are likely to account for differences in the published results of overlapping TAXUS and CYPHER stents. The differing dose-response curves and the pharmacokinetics of the available drugs may have variable effects on the local vascular biology, so that it may not be possible to extrapolate the results from one drug-eluting stent to another.
Study limitations
Although this is the largest randomised trial in which overlapping DES have been subject to detailed assessment (including follow-up angiography and intravascular ultrasound), patients are necessarily selected, and the trial is underpowered to formally assess some endpoints including the incidence of stent thrombosis. Furthermore, there was a non-random assignment to the overlapping stent study groups. Finally, clinical follow-up is short and therefore no comment can be made in relation to late safety and efficacy.
Conclusions
An overlap strategy using multiple polymer based paclitaxel eluting TAXUS MR stents was effective in reducing clinically relevant restenosis compared with a bare metal stent control. Overlapping TAXUS MR stents were not associated with an increase in nine month MACE, late acquired aneurysm formation, late acquired incomplete stent apposition or late stent thrombosis. The higher peri-procedural non-Q wave myocardial infarct rate in the TAXUS group indicates the need for ongoing vigilance particularly in relation to side branch compromise which may be more common with polymer based DES.
Contributors
Keith D. Dawkins and Eberhard Grube as principal investigators were responsible for the conception, design and execution of the study, the analysis and interpretation of the data, and the writing of the manuscript. Drs Giulio Guagliumi, Adrian Banning, Krzysztof Zmudka, Antonio Colombo, Leif Thuesen, Karl Hauptman, Jean Marco, William Wijns, Jeffrey J Popma, substantially participated in enrolment of patients, execution of the study, and the review of the manuscript. Mary E. Russell and Joerg Koglin participated in the design and execution of the study, the analysis of the data, and contributed to the writing of the manuscript. All authors approved the final version of the report. With thanks to Gary Jasdzewski PhD who assisted in the preparation of the manuscript.
Clinical events committee
Donald E. Cutlip, (Chairman), Kalon Ho, Joseph Kannam, Julian Aroesty, Boston, USA, Manish Chauhan , Burlington, USA, Michel Vandormael, Bay Pines, USA, Germano Di Sciascio, Rome, Italy.
Data safety and monitoring board
Michael Haude (Chairman), Essen, Germany, Gerhard Bauriedel, Bonn, Germany, Bernard Meier, Bern, Switzerland, Wolfgang Köpcke, Munster, Germany.

