Abstract
Aims: Carotid artery stenting (CAS) prior to open-heart surgery may be a useful approach to minimise the risk of neurologic events in patients undergoing aortic valve replacement (AVR).
Methods and results: All patients referred for carotid intervention at our institution between 1998 and 2005 with concomitant severe aortic stenosis (AS) (aortic valve area <1.0 cm2) were included. Data were obtained prospectively and confirmed by chart review. The primary endpoint was all-cause mortality at 30-days after CAS or AVR. Secondary endpoints included incidence of stroke, transient ischaemic attack (TIA), and myocardial infarction (MI) at 30-days after CAS or AVR. Patients were followed-up at 30-days, six months, and annually thereafter. Of the 829 patients who underwent CAS, 52 had severe AS. Carotid stenting in 28 (54%) of the patients was attempted using embolic protection devices. Three patients (6%) died <30 days after carotid stenting, and two (4%) died >30 days after carotid stenting but prior to aortic valve replacement. At one year after CAS, a total of nine patients had died. There were a total of 19 deaths (37%) over a median follow-up of 3.8 years. One patient (2%) suffered a TIA during carotid stenting; at 30-days and 1-year there were no strokes in the CAS group. There were no MI’s. AVR was performed in 29 patients (56%), and at 30-days and 1-year there were no strokes or deaths noted in those patients.
Conclusions: In patients with severe AS, CAS can be accomplished effectively and with a low rate of stroke, MI, and death.
Introduction
Atherosclerotic disease and degenerative AS share many etiologic risk factors including older age, hypertension, and hyperlipidaemia1. The incidence of carotid stenosis (CS) is estimated at 13% in patients with isolated degenerative AS and up to 27% in patients with degenerative AS and coronary artery disease (CAD)2,3. As a result, the use of Doppler ultrasound to screen for CS in patients with AS prior to AVR has become commonplace4,5.
In patients undergoing open heart surgery, the presence of carotid stenosis (CS) significantly increases the risk of perioperative stroke6. In patients with asymptomatic carotid stenosis, the stroke rate after coronary artery bypass grafting may be as high as 11%7. Once diagnosed, the management options for CS in this setting are controversial, but traditionally include either staged or simultaneous carotid endarterectomy (CEA) and cardiac surgery8,9. However, both of these strategies carry a high risk of post-operative stroke and mortality10,11.
In recent years, the use of carotid artery stenting (CAS) with embolic protection devices (EPD’s) has proven successful in patients with CS who are considered “high-risk” for CEA12. Furthermore, the use of CAS in patients prior to CABG has shown similar, and in some cases better, results when compared with staged or simultaneous CEA13,14. Versaci and colleagues recently provided the results of their prospective trial of “hybrid” CAS followed immediately by on-pump CABG15. In a group of 101 patients, the combined risk of MI, stroke, and death was 4%, which was quite favourable when compared to studies of staged CEA-CABG. As experience and evidence grows, the application of CAS prior to open heart surgery (OHS) is expected to increase.
The use of CAS in patients with severe AS raises many concerns, most often related to the haemodynamic challenges inherent to this valvular lesion. To our knowledge, however, there has been no systematic study of CAS in this patient population. The aim of our paper is to establish the safety and efficacy of carotid artery stenting in patients with severe aortic stenosis.
Methods
Patient population
Between 1998 and 2005, a total of 829 consecutive patients in the Carotid Artery Intervention Registry at our institution were referred for carotid stenting, of whom 52 (6%) had severe aortic stenosis (aortic valve area ≤1.0 cm2). All patients who underwent carotid stenting at our institution were followed by an Institutional Review Board approved carotid stent registry and all patients provided informed consent. Patient demographics, clinical parameters, interventional procedures, procedural outcomes, and complications were recorded prospectively for all patients. Outpatient follow-up was scheduled at 30 days, six months, and annually thereafter.
Briefly, all patients with severe AS and symptomatic carotid stenosis ≥60% or asymptomatic carotid stenosis ≥80% underwent carotid angioplasty followed by carotid stenting. Embolic protection devices (EPDs) were used in 28 patients (54%). The carotid procedure was performed using unfractionated heparin (UFH) to achieve an ACT of >250 seconds. During the early years of the procedure our standard included the intraprocedural administration of glycoprotein IIb/IIIa inhibitors, which was given in 20 (38%) of the cases. This practice has since been found unfavourable compared with use of EPDs16. Dual antiplatelet therapy (aspirin plus clopidogrel) was continued for four weeks after CAS, followed by aspirin only thereafter. Three patients underwent AVR <15 days after CAS. In these patients clopidogrel was discontinued five days before AVR, eptifibatide bridge was started three days prior to AVR and discontinued on the morning of surgery, and clopidogrel was resumed immediately after surgery with a loading dose of 300 mg.
Carotid stenosis was assessed with carotid ultrasound using the modified Strandness criteria17. Symptomatic stenosis was defined as a history of TIA or stroke; asymptomatic disease was defined as a lack of these features. Severe aortic stenosis was defined as an aortic valve area (AVA) of less than or equal to 1.0 cm2 as measured by transthoracic echocardiography18. Though all patients had a calculated aortic valve area of <1.0 cm2, a number of patients had a mean gradient below 40 mmHg in the setting of LV systolic dysfunction. These patients were confirmed to have severe AS based on the appearance of the valve, planimetry, and/or the dimensionless index as appropriate. All echocardiograms were evaluated by a cardiovascular imaging specialist.
Procedural complications and haemodynamic assessments were prospectively collected during the procedure. Our primary endpoint was all-cause mortality, at a median follow-up of 3.8 years. Secondary endpoints were the incidence of TIA, stroke, or myocardial infarction (defined as CK-MB fraction >10 ng/mL and >5% of the total CK; or troponin-T >0.10 ng/mL) at 30 days after CAS only or 30 days after AVR as appropriate. In addition, the incidence of AVR after carotid stenting was determined. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and were analysed using the t-test. Categorical variables were analysed using the Chi-square test. Unadjusted Kaplan-Meier curves assessed the difference in mortality by age and whether patients underwent AVR.
Results
Patient characteristics
The baseline demographic characteristics of the study cohort are shown in Table 1.
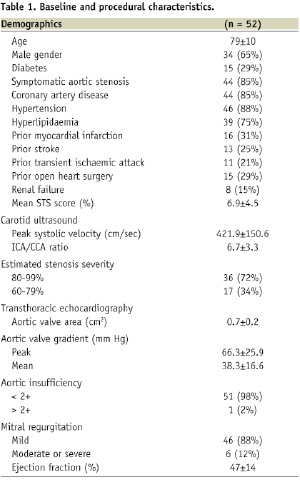
Of the 52 patients in our study, most had symptomatic aortic stenosis and almost half had symptomatic carotid stenosis. In addition to severe aortic stenosis many patients had significant other comorbidities, yielding a mean STS score of 6.9±4.5%19. Baseline carotid ultrasound parameters are also shown in Table 1. Twenty-eight patients had right-sided carotid stenosis (54%) and twenty two patients (42%) had left-sided carotid stenosis.
Baseline echocardiographic data are reported in Table 1. The mean aortic valve area (AVA) for the group was 0.7±0.2 cm2 and the AVA was <0.7 cm2 in 18 patients (35%). The average ejection fraction (EF) for the group was 47±14%. The EF was normal (>50%) in 26 patients (50%) and markedly depressed (<35%) in 13 patients (25%). Six patients (12%) had severely depressed EF (≤35%) and severe aortic stenosis with AVA ≤0.7 cm2.
Procedural characteristics
Carotid artery angioplasty or stenting was successful in 98% of the patients (Table 2).
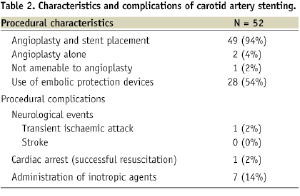
One patient (2%) was not amenable to angioplasty due to severely calcified, subtotal occlusion of the right internal carotid artery and robust collateral filling of the intracerebral circulation from the left. Angioplasty alone was performed in two patients (4%) because AVR was scheduled within the following days and the operators did not yet have a clear antiplatelet bridging regimen. The procedure was performed using embolic protection devices in 28 patients (54%) for whom angioplasty was possible.
Seven patients (13%) had Swan-Ganz catheters placed for haemodynamic monitoring during the procedure. These patients were chosen to have haemodynamic guidance a priori due to either severe LV dysfunction (EF <35%) or the presence of clinical heart failure at the time of the procedure.
Procedural complications
Procedural complications at the time of CAS included: one patient (2%) who suffered a TIA (patient characteristics: AVA 0.8 cm2; 90% right internal carotid artery stenosis; EF 60%; EPD was not used in this patient) and one patient (2%) who developed ventricular tachycardia and fibrillation and was successfully resuscitated (patient characteristics: AVA 0.7 cm2, EF 15%). Seven patients (13%) required norepinephrine administration for hypotension during the procedure, and one patient (2%) required less than 24 hours of continued dopamine infusion for postprocedural hypotension. One patient developed haemoptysis post-CAS in the setting of dual antiplatelet therapy as well as UFH drip which was started for bridging to warfarin due to a history of atrial fibrillation. Bronchoscopy was performed and did not reveal any focal lesions. The bleeding resolved with conservative management and discontinuation of anticoagulation (Table 2).
Procedural outcome
The outcome and complications of CAS are grouped on the basis of symptomatic status of CS and AS in Table 3 and are described below.
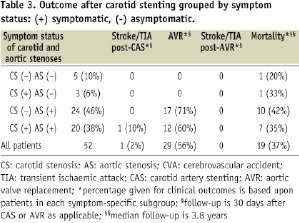
Clinical events are reported at 30 days after CAS only or AVR as applicable. Mortality data is reported with a median follow-up of 3.8 years. Mortality data was compiled using chart review; cause of death and chronicity of illness prior to death was therefore not ascertainable for all patients.
GROUPED BY AS SYMPTOM STATUS
In our cohort, a total of eight patients (16%) who underwent CAS had asymptomatic AS, five of whom also had asymptomatic CS. None of these patients underwent AVR. Two patients died of an undetermined cause >30 days after CAS. There were no cerebrovascular events post-CAS. A total of 44 patients (84%) had symptomatic AS, 20 of whom also had symptomatic CS. During carotid stenting, one patient (2%) developed transient aphasia with complete resolution (TIA). A total of 29 of these patients underwent AVR after CAS. At a median follow-up of 3.8 years, 19 patients died.
GROUPED BY CS SYMPTOM STATUS
Our cohort included 29 patients (56%) with asymptomatic CS, 24 of whom had symptomatic AS. None of these patients suffered a neurologic complication from CAS. A total of 17 patients underwent AVR and 11 patients had died at 3.8 years of follow-up. Twenty-three patients (44%) had symptomatic carotid disease, 20 of whom had symptomatic AS. During carotid stenting, one patient (5%) developed transient aphasia with complete resolution (TIA). Twelve of the patients underwent AVR, and a total of eight patients died at follow-up.
Management of aortic stenosis after carotid stenting
AVR was performed in 29 patients (56%), of whom 26 (50%) underwent AVR ≥30 days after carotid stenting and three patients (6%) had AVR <15 days after carotid stenting (with eptifibatide bridge). The remaining 23 patients (44%) did not undergo AVR (Figure 1).
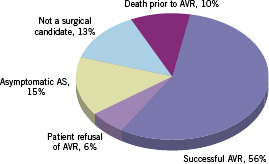
Figure 1. Management of severe aortic stenosis after successful carotid artery stenting.
Five patients (10%) died prior to AVR. Three patients (6%) expired <30 days after CAS and prior to AVR: two with decompensated heart failure (EF≤15%) and ventricular arrhythmia, and one due to pulmonary embolism (with normal EF). Two other patients (4%) expired >30 days after carotid stenting but prior to AVR, both with decompensated heart failure. Both patients were high-risk surgical candidates with EF≤45.
Three patients (6%) had balloon aortic valvuloplasty as a bridge to potential AVR. Of these, two (4%) were high-risk surgical candidates with EF≤35 who expired without an AVR (STS mortality score of 17% and 5%). The remaining patient had a successful AVR with two vessel bypass surgery 52 days after balloon aortic valvuloplasty.
Of the 29 patients (56%) who underwent AVR, 22 (76%) had concomitant coronary artery bypass grafting.
Outcome after AVR
At the 30 day follow-up after surgery, none of the 29 patients who had undergone AVR had CVA, TIA, or death. We also found that patients who underwent AVR after CAS had improved survival compared with those who did not have AVR (Figure 2).
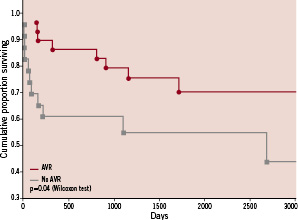
Figure 2. Survival among patients with severe aortic stenosis who undergo successful carotid artery stenting is improved by aortic valve replacement.
Discussion
Although revascularisation with CEA has been widely accepted as a strategy to minimise the neurologic complications associated with OHS in patients with severe carotid stenosis, it is not frequently used. Strategies of staged or simultaneous CEA and OHS have been evaluated in several studies in the past8-11. Unfortunately, even these studies of highly selected patients with low or moderate risk have shown a variable but high rate of post-operative stroke (up to 6.4%), MI (up to 6.5%), and death (up to 12.9%) in the short-term. Therefore, carotid stenting prior to OHS for severe carotid stenosis, whether clinically evident or asymptomatic, may be an important treatment option for these patients.
The use of carotid artery stenting has been shown to be non-inferior to CEA in patients who are at high risk for carotid surgery, and prospective trials comparing CAS to CEA in lower-risk patients are ongoing20,21. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial was designed to evaluate the non-inferiority of carotid artery stenting with embolic protection versus CEA in high-risk patients22. At one year of follow-up, the incidence of the primary endpoint (death, stroke, or myocardial infarction within 30 days or death or ipsilateral stroke between 31 days and one year) was 12% after CAS vs. 20% after CEA (p=0.004 for non-inferiority and p=0.053 for superiority). Long-term (3-year) outcomes in this patient group were recently reported, with continued evidence of the non-inferiority of CAS (stroke rate 9.0% in both groups)12.
Given its potential for reducing neurologic and cardiac complications in patients with carotid stenosis, carotid stenting with embolic protection devices has also been studied as a staged procedure prior to OHS. Ziada and colleagues evaluated outcomes in 64 patients with both severe carotid stenosis and coronary atherosclerosis treated with carotid stenting followed by OHS and compared them to 112 patients who underwent combined CEA and OHS23. Although there was no difference in mortality, the stented patients had a significantly lower incidence of stroke (2% vs. 9%, p=0.05) and stroke or MI (6% vs. 19%, p=0.02). It is important to emphasise that this was despite a higher incidence of unstable angina, systolic dysfunction, critical aortic valve stenosis, TIA, stroke, or history of previous OHS in the CAS group.
Van der Heyden and colleagues provided their experience on CAS prior to CABG in 356 patients with severe asymptomatic carotid stenosis24. In the period between CAS and 30-days after CABG, the rates of stroke, TIA, MI, and death were 3.1%, 3.7%, 2.0%, and 3.7%, respectively. Five-year event rates were similar to those seen in the short-term follow-up, providing evidence for the continued safety and efficacy of this management strategy. Taken together, these studies show that staged CAS and CABG is not only safe and effective from a neurologic perspective, but also serves to minimise the perioperative cardiac risks that occur in a CEA plus CABG strategy.
Patients with aortic stenosis pose a significant challenge for carotid revascularisation. Since these patients typically can not tolerate abrupt preload reduction, hypotension following carotid stenting can be dangerous. With regard to the haemodynamic safety of the procedure in our cohort, one patient (2%) experienced cardiac arrest during the case and was successfully resuscitated. It is likely that the haemodynamic disruptions of carotid sinus stimulation contributed to the arrest. Eight patients (15%) experienced hypotension that required the transient use of vasopressor agents, an adverse event that is widely recognised during CAS in patients both with and without AS25. We used a pulmonary artery catheter to provide invasive haemodynamic monitoring in seven patients (13%). Our study shows that in an experienced centre, with careful medical management, carotid stenting can be safely performed in this group of patients.
There are of course logistical concerns regarding the need for dual antiplatelet therapy (aspirin plus clopidogrel) after CAS in patients with pending cardiac surgery. Antiplatelet therapy increases the risk for re-exploration for bleeding and transfusion in patients undergoing cardiac surgery26,27. Therefore, it is preferable to delay OHS for 2-4 weeks after CAS, after which time clopidogrel (and if necessary, aspirin) may be withheld for one week28. In situations where this strategy is not feasible, alternative strategies include: direct transfer from CAS to OHS using aspirin and unfractionated heparin, use of heparin and glycoprotein IIb/IIIa inhibitors as a “bridge” during OHS, or combined CAS and OHS29. A recent feasibility study of “hybrid” or simultaneous CAS and CABG was reported by Palombo and colleagues14. In a group of 22 patients, the strategy resulted in no myocardial infarctions and no ipsilateral strokes, suggesting this to be a safe and effective approach. Similarly, Versaci and colleagues recently reported encouraging results of their prospective trial of 101 high-risk patients undergoing hybrid CAS-CABG with a 4% risk of the combined endpoint (stroke, MI, death)15. Aortic valvuloplasty as a “bridge” in those patients who cannot wait for AVR due to haemodynamic instability is also a potential strategy and was used in three patients in our series.
Another important finding of our study, similar to that of previous investigators, is that many high-risk patients with severe AS do not undergo surgical AVR (Figure 1). In fact, only 56% of our patients underwent AVR. While all of our patients were initially referred for CAS with the expectation of AVR, a substantial number did not ultimately have surgery for a number of reasons including patient preference and prohibitive surgical risk. With the increasing availability of transcatheter aortic valve implantation (TAVI), many of these patients may be offered percutaneous management of their AS. Even without AVR, however, carotid revascularisation may still be beneficial in the patients with asymptomatic severe carotid stenosis when performed with a reasonable complication rate, and clinical trials of CAS in these high-risk patients are currently ongoing30,31.
Limitations
Though clinical events were collected prospectively in the carotid stenting database, we did conduct a retrospective chart review with all of the limitations inherent to the same. Our study includes a small number of patients and therefore, while the results are encouraging, can still only be considered hypothesis-generating. We did not have a control group, and therefore cannot compare the strategy of CAS in the setting of severe aortic stenosis with CEA. However, given the success of carotid stenting in patients with aortic stenosis at our institution, many patients presenting with this scenario have been referred for percutaneous treatment in the last several years. Lastly, our study is from a single centre with experienced operators; therefore, it may not be applicable to other settings.
Conclusion
The data provided here are, to our knowledge, the first thorough report of carotid stenting in patients with severe AS. Protected carotid stenting is a valuable strategy in the management of patients with severe carotid disease and severe aortic stenosis. Recent developments in transcatheter aortic valve implantation (TAVI) heralds a new era for elderly patients with multiple comorbidities who have severe AS and severe carotid disease. Future studies may assess the safety and efficacy of a completely percutaneous approach (CAS followed by TAVI) for improving the outcome of patients who are at high risk for traditional AVR despite successful carotid stenting. Ultimately, a close collaboration between peripheral and structural interventionalists as well as cardiac surgeons is imperative in developing a cohesive, multidisciplinary plan of care for these high-risk patients.

