Abstract
Background: The A1166C polymorphism of the angiotensin II type 1 receptor (AT1R) gene, which appears to be the main receptor mediating the pleitropic vascular effects of angiotensin II in human beings, has been associated with the risk of myocardial infarction (MI), the severity of coronary vasoconstriction and the occurrence of sudden death. The question therefore arises whether the A1166C polymorphism constitutes a hereditary risk factor of survival after an acute myocardial infarction. Methods and results: In a large prospective study of 970 consecutive patients with a recent myocardial infarction the A1166C polymorphism was detected using a PCR based protocol. During the follow-up period (median, 2.5 years), 75 patients died and 62 from cardiovascular causes. The prespecified primary and secondary end points considered were total mortality and cardiovascular mortality. No differences between AA, AC, and CC groups were observed with respect to baseline clinical characteristics. Beyond conventional risk factors like age (RR=2.8 [1.6-5.0] 95% CI; p<0.001), hypercholesterolemia (RR=2.1 [1.2-3.7] 95% CI; p<0.014) or low left ventricular ejection fraction (RR=2.7 [1.5-4.8] 95% CI; p<0.002), the AT1R CC genotype was also identified as a strong independent predictor of death after myocardial infarction (RR=3.2 [1.5-7.0] 95% CI ; p<0.004). After adjustment for mortality causes, the AT1R CC genotype was confirmed to be an independent predictor of cardiovascular death after myocardial infarction (RR=2.8 [1.2-6.5] 95% CI; p<0.021). Conclusions: The AT1R CC genotype after adjustment is a strong predictor of death in post MI patients. This new finding may help to identify high risk post MI patients who may benefit from new therapeutic strategies.
Introduction
Since the renin-angiotensin system (RAS) has been confirmed to be involved in the pathogenesis of atherosclerosis1, several reports have focused on the association of polymorphisms of the RAS and different aspects of coronary artery disease. Angiotensin II, generated at the end of the enzymatic cascade of the RAS is a powerful vasoconstrictor, a growth-promoting factor for vascular smooth muscle cells and can contribute to arterial thrombosis through plasminogen activator inhibitor-1 up-regulation2,3,4,5,6. All these effects can be critical for the occurrence of major adverse cardiac events (MACE) in patients with established coronary artery disease and especially in patients with recent acute myocardial infarction (MI). It has been suggested that the A1166C polymorphism of the angiotensin II type 1 receptor (AT1R) gene, which appears to be the main receptor mediating the pleitropic effects of angiotensin II in human beings, could be associated with the severity of coronary vasoconstriction7, the risk of MI8 and the occurrence of sudden death9. The question therefore arises whether the A1166C polymorphism constitutes a hereditary risk factor of survival after an acute myocardial infarction. Some contradictory results exist regarding the role of the A1166C polymorphism as an atherosclerotic risk factor but no data is available on the long term follow-up of patients at high risk of cardiovascular events and bearing the C allele of the AT1R. The potential role of a genetic variant of the AT1R gene as a pathogenetic factor in the occurrence of cardiovascular death after an acute myocardial infarction (MI) is of particular interest given the large number of patients who are treated around the world for this pathology and because of the ready availability of target-specific pharmacologic agents in the form of angiotensin II receptors blockers. Clearly the clinical implications of this finding would be more far-reaching if the AT1R genotype not only was associated with the occurrence of death after MI, but also represented an identifiable and modifiable risk factor for secondary prevention.
Methods
Patient population
From November 1996 to July 2000, 970 consecutive Caucasian patients with an acute or recent transmural myocardial infarction, according to the ACC/AHA definition10, undergoing cardiac catheterization and successful coronary stenting of the culprit lesion were enrolled in this study. All electrocardiograms were reviewed to ensure the diagnosis of transmural MI following the acute phase. Blood sampling was performed at the time of the invasive procedure after an informed consent was obtained.
Endpoints and definitions
This prospective observational study was designed to examine the relationship between the A1166C polymorphism of the angiotensin II type 1 receptor gene and the risk of death after an acute myocardial infarction. The pre-specified primary and secondary endpoints considered were total mortality and cardiovascular mortality. Autopsies were not systematically performed and death was regarded as cardiac except for those of proven non cardiac origin. Especially sudden or unexpected deaths were classified as cardiovascular death. Follow-up was obtained by mailed questionnaires and scripted telephone interviews performed by a physician. Events were verified by contacting the patients’ primary physician and reviewing medical records and death certificates.
Genetic analysis
Genomic DNA from each patient was prepared from peripheral leucocytes by salts precipitation method. Polymorphism A1166C in the untranslated 3’ part of the AT1 R gene was determined using the palindromic sequence created by the mutation. A specific enzyme (Dde 1 enzyme) cleaves the PCR product in presence of the mutation. The PCR primers were chosen to obtain by electrophoresis, in case of mutation, two fragments of different sizes easy to visualize on metaphore gel. Amplification product measures 350 bp and after 4 hours of digestion, the 1166 A allele is not cleaved whereas the 1166 C allele generates two fragments of 237 and 113 bp11,12.
Statistical analysis
Base-line characteristics of the study population are presented as counts and percents for categorical variables and as mean ± SD for continuous variables. Differences in percents were evaluated by the Chi-square test, and means by ANOVA. Validity conditions were checked for each comparison. No striking deviation from Hardy-Weinberg equilibrium was observed for the distribution of AT1R A/C gene polymorphism. A univariate analysis with the Kaplan-Meier product-limit method and the log rank or Breslow tests was used to compare the different groups of genotypes.
For the identification of independent prognostic factors related to total mortality and cardiovascular death Cox’s proportional hazards models were used after adjustment for variables proved to be significant with univariate statistical analysis (P<0.20). The relative risks were given with a 95 percent confidence interval (95% CI). All calculations were performed with SPSS Professional Statistics™ 10.0.7 (SPSS Inc., Chicago, Illinois).
Results
Overall genotype frequencies for wild type (AA), heterozygous (AC) and homozygous (CC) polymorphisms were 502 (52%), 386 (40%), and 82 (8%). These findings were in accordance with the Hardy-Weinberg equilibrium. No differences between AA, AC, and CC groups were observed with respect to baseline clinical characteristics including risk factors or use of ACE-I (angiotensin converting enzyme-inhibitor) or ARB (angiotensin receptor blockers) (Table 1). Similar left ventricular ejection fractions were observed for the three groups of genotypes. Severity of coronary artery disease was also similar in the three groups of genotypes. During the period of follow-up, 14 non-fatal MI occurred without statistical significant difference between the groups. Coronary artery bypass surgery was performed for 29 patients and 136 patients underwent a new PCI during the period of follow-up with no difference among the three groups of genotypes.
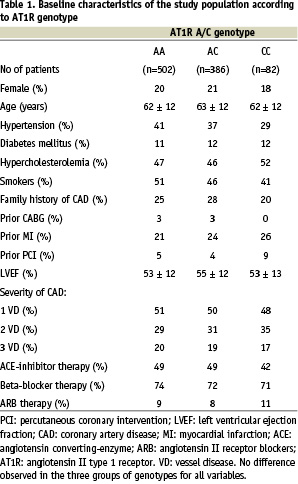
Clinical follow-up information concerning the mortality was obtained for 968 patients (99.8%). A mean follow-up period of 2.5 years was ensured for the whole study population. Among the 75 deaths observed during the follow-up period, 62 (83%) were related to a cardiovascular cause and 13 (17%) not related to heart disease. According to the genotypes the cardiovascular mortality rates were 6.4%, 5.1% and 12.3% for AA, AC and CC patients respectively. The pre-specified end-points of the study were related to the presence of the CC genotype as shown by the Kaplan-Meier analyses (Figures 1 and 2).
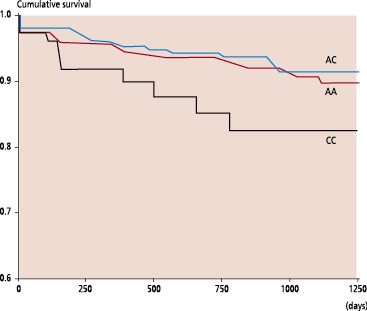
Fig. 1: Kaplan-Meier estimates of death in relation to the AT1R genotypes (P log-rank = 0.07)
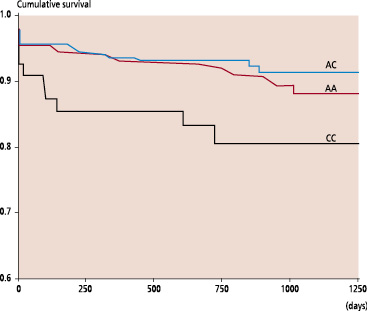
Fig. 2: Kaplan-Meier estimates of cardiovascular death in relation to AT1R genotypes (P log-rank = 0.014)
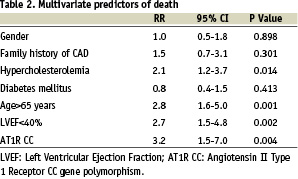
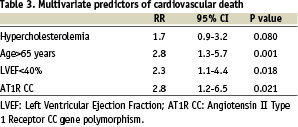
Were included in the multivariate analyses the following variables (p value < 0.2 by univariate analysis): hypertension, diabetes, gender, hypercholesterolemia, family history of CAD, age>65 years, poor LVEF<40%, severity of the CAD and AT1 CC gene polymorphism. The relative risks and 95 percent confidence intervals (95% CI) derived from the Cox regression analysis are given for adjusted predictors of death in Table 2. Beyond conventional risk factors like age (RR=2.8 [1.6-5.0] 95% CI; p<0.001), hypercholesterolemia (RR=2.1 [1.2-3.7] 95% CI; p<0.014) or low left ventricular ejection fraction (RR=2.7 [1.5-4.8] 95% CI; p<0.002), the AT1R CC genotype was identified as a strong independent predictor of death after myocardial infarction (RR=3.2 [1.5-7.0] 95% CI; p<0.004). After adjustment for mortality causes, the AT1R CC genotype was confirmed as an independent predictor of cardiovascular death after myocardial infarction (RR=2.8 [1.2-6.5] 95% CI; p<0.021) (Table 3).
Discussion
This report is the first large prospective study investigating the influence of the AT1R gene polymorphism on the long term clinical follow-up after MI and showing a strong influence of the A1166C mutation on the survival after myocardial infarction.
The central position of the AT1R means it is plausible that genetically determined changes in the coding and /or non-coding regions of the receptor may play a role in the development of cardiovascular disease. The rationale for investigation of this question is the fact that angiotensin II is not only a growth-promoting factor for vascular smooth muscle cells and a potent vasoconstrictor but also apparently a prothrombotic substance2,3,4,5,6. At the molecular and cellular levels angiotensin II is able to promote the initiation and the progression of atherosclerosis13,14,15 and to reduce efficiency of the fibrinolytic system through the production of plasminogen activator inhibitor-1 in vascular tissue4,5,6.
Association of the A1166C polymorphism and the risk of MI remains controversial8. Recently the A1166C AT1R gene polymorphism located in the 3’ untranslated region of the gene has also been associated with malignant ventricular arrhythmias9 and the severity of coronary artery vasoconstriction in humans7. In accordance with this latter finding in vitro studies have demonstrated that the AT1R gene polymorphism is associated with an increased vasoreactivity to angiotensin II in human arteries16. All these deleterious effects are compatible with the pleitropic vascular effects of angiotensin II and may participate in the increased risk of death in a high risk population like patients with a history of MI.
The higher risk of cardiovascular death was restricted to subjects homozygous for the C allele of the AT1R gene polymorphism suggesting a recessive effect. This observation is consistent with previous studies showing a recessive effect of this gene polymorphism namely on coronary vasomotion in humans7. Given the low frequency of the C allele and CC genotypes in human populations, further investigations are needed to confirm these results and to clarify the possible mechanisms involved in this genetic susceptibility risk factor of death after MI. In particular it is important to differentiate between a direct but less probable role of the AT1R A1166C mutation and a strong linkage disequilibrium of this polymorphism with a causal variant. In the AT1R gene, several polymorphisms have been identified and none of these polymorphisms appeared to be functional17,18,19 but the A1166C transversion has been associated with various cardiovascular diseases8,17. Therefore it was assumed that this polymorphism might be linked to a yet unidentified functional variant. The AT1R A1166C gene polymorphism is highly suspected to be associated with functional alterations in the responsiveness of the G-protein-coupled receptor to angiotensin II as supported by both in vivo and in vitro studies in human arteries7,16 with a potential increased agonist responsiveness. Indeed greater coronary vasoconstriction in humans has been associated with the presence of the AT1R CC genotype7,16. Furthermore localized segmental hyper-reactivity in the infarct-related vessel has been observed in 20% of patients with recent myocardial infarction20. Therefore the deleterious effect of the AT1R CC genotype on coronary artery vasomotion especially after MI could explain the increased cardiovascular death observed in the present study.
It would be especially interesting to find out whether an association of the AT1R C allele to survival could be detected in the large recently published trials testing for angiotensin II receptor blockers in post MI patients21,22,23,24 and obviously to search for potential interactions of these agents with this genetic susceptibility risk factor of death after MI.
In conclusion, our findings demonstrate that the AT1R A1166C mutation may affect the patient outcome after MI, with an increased risk of cardiovascular death in CC individuals. Therefore the A1166C AT1R gene polymorphism might be a useful marker for assessing the long-term risk of death after MI and for developing new secondary prevention strategies. However as previously mentioned, given the low rate of patients homozygous for the A1166C mutation of the AT1R gene further investigations are needed to confirm our results.
Acknowledgments
The authors thank the cardiac catheterization laboratory staff and all the interventional cardiologists for their support and assistance, without which this study would not have been possible.

