Abstract
Aims: Angiographic parameters (such as late luminal loss) are common endpoints in drug-eluting stent trials, but their correlation with the neointimal process and their reliability in predicting restenosis are debated.
Methods and results: Using quantitative coronary angiography (QCA) data (49 bare metal stent and 44 sirolimus-eluting stent lesions) and intravascular ultrasound (IVUS) data (39 bare metal stent and 34 sirolimus-eluting stent lesions) from the randomised Reduction of Restenosis In Saphenous vein grafts with Cypher stent (RRISC) trial, we analysed the “relocation phenomenon” of QCA-based in-stent minimal luminal diameter (MLD) between post-procedure and follow-up and we correlated QCA-based and IVUS-based restenotic parameters in stented saphenous vein grafts. We expected the presence of MLD relocation for low late loss values, as MLD can “migrate” along the stent if minimal re-narrowing occurs, while we anticipated follow-up MLD to be located close to post-procedural MLD position for higher late loss.
QCA-based MLD relocation occurred frequently: the site of MLD shifted from post-procedure to follow-up an “absolute” distance of 5.8 mm [2.5-10.2] and a “relative” value of 29% [10-46]. MLD relocation failed to correlate with in-stent late loss (rho=0.14 for “absolute” MLD relocation [p=0.17], and rho=0.03 for “relative” relocation [p=0.81]). Follow-up QCA-based and IVUS-based MLD values well correlated in the overall population (rho=0.76, p<0.001), but QCA underestimated MLD on average 0.55±0.49 mm, and this was mainly evident for lower MLD values. Conversely, the location of QCA-based MLD failed to correlate with the location of IVUS-based MLD (rho=0.01 for “absolute” values – in mm [p=0.91], rho=0.19 for “relative” values – in % [p=0.11]). Overall, the ability of late loss to “predict” IVUS parameters of restenosis (maximum neointimal hyperplasia diameter, neointimal hyperplasia index and maximum neointimal hyperplasia area) was moderate (rho between 0.46 and 0.54 for the 3 IVUS parameters).
Conclusions: These findings suggest the need for a critical re-evaluation of angiographic parameters (such as late loss) as endpoints for drug-eluting stent trials and the use of more precise techniques to describe accurately and properly the restenotic process.
Introduction
Quantitative coronary angiography (QCA) has become a trusted tool to evaluate the restenotic process and the relative efficacy of percutaneous interventions1,2 and it has been routinely used to define endpoints in recent trials involving drug-eluting stents3-7. One of the most commonly used angiographic endpoints is late luminal loss, which is the difference between post-procedure and follow-up minimal luminal diameter (MLD). This parameter has several limitations. The accuracy of the correlation between late loss and the neointimal restenotic process is still debated8-10 and its reliability in predicting restenosis and repeated revascularisation is also questioned3,11-15. Moreover, as late loss is calculated regardless of the respective axial location of the MLD along the stent between post-procedural and follow-up QCA analysis, MLD relocation can be another important technical limitation affecting the value of late loss16,17.
All the data presented in the aforementioned referenced publications were analysed in studies performed in native coronary arteries, but no data are available in diseased saphenous vein grafts (SVG), which can be used as a good model for QCA analysis because of the lack of side branches and the limited probability of overlap with other vessels (despite the possible presence of surgical clips and wires, which in any case are easily recognisable at QCA).
Using QCA and intravascular ultrasound (IVUS) data from the Reduction of Restenosis In Saphenous vein grafts with Cypher stent (RRISC) trial, which randomly compared sirolimus eluting stent versus bare metal stent in SVG18, we comprehensively assessed values and drawbacks of late loss. In particular, we analysed the “relocation phenomenon” of MLD and we focused on its possible correlation with the degree of late loss formation. On one hand, we expected low values of late loss associated with potentially significant MLD relocation, as MLD can easily “migrate” along the stent maintaining similar absolute post-procedural and follow-up values if minimal in-stent re-narrowing occurs. On the other hand, for high late loss values we anticipated follow-up MLD to be located close to the post-procedural MLD position, as post-procedural MLD is known to be a powerful predictor of restenosis19. We further correlated QCA-based and IVUS-based restenotic parameters in stented SVG.
Methods
Population selection
The RRISC is a randomised, double blind, trial. Its design and results have been previously published18,20. Briefly, 75 patients with a history of previous coronary artery bypass surgery and with 96 de novo target lesions localised in 80 diseased SVG with a reference vessel diameter >2.5 and <4.0 mm, were included. Patients were randomly allocated in a 1:1 ratio to treatment with Cypher sirolimus-eluting stent or BX-Velocity bare metal stent (both from Cordis, Johnson & Johnson, Miami Lakes, FL, USA).
Coronary angiography was repeated at six months (±15 days) and IVUS analysis was recommended, only during follow-up angiography, in every SVG treated with a study stent. IVUS was performed after injection of 0.2 mg of nitroglycerin with a 30 MHz ultrasound probe (Ultracross 2.9F, Boston Scientific Corporation, Natik, MA, USA), connected to the Galaxy ultrasound console (Boston Scientific Corporation, Natik, MA, USA), and a motorised pullback (speed: 0.5 mm/sec).
QCA analysis
Digital coronary angiograms were analysed offline by an independent expert, not performing any of the procedures and blinded to treatment allocation, using a validated automated edge detection system (CAAS II, PIE Medical, Maastricht, The Netherlands). His intra-observer variability for QCA measurements is <5%. Matched views were selected for angiograms recorded before the intervention, immediately after and at 6-month follow-up. A single monoplane view was analysed per lesion treated, specifically the least foreshortened one was selected. Angiographic measurements were made in the stent during diastole using the contrast-filled guiding catheter for magnification calibration. In case overlapping stents were placed, a single in-stent value was measured. MLD was evaluated at baseline, at the end of the procedure and at 6-month follow-up. Binary angiographic restenosis was defined as a diameter stenosis >50% at follow-up control angiography. Late lumen loss was calculated as the difference in MLD between measurements immediately after the procedure and at follow-up. We defined the position of MLD in the stent as the distance of MLD from the proximal edge of the stent, either post-procedure and at follow-up. The difference between post-procedural MLD position and follow-up MLD position was the “absolute” relocation of MLD (in mm). In order to overcome partially the possible errors in QCA measurement due to vessel foreshortening, we defined also the “relative” position of MLD in the stent as the ratio, expressed as percentage, between the distance of MLD from the proximal edge of the stent and the total length of the stent, either post-procedure and at follow-up. The difference between post-procedural “relative” MLD position and follow-up “relative” MLD position was the “relative” relocation of MLD, expressed as a percentage.

We decided to analyse only in-stent and not in-segment parameters, because in-stent late loss has been shown to correlate better with binary restenosis than in-segment late loss3, and it is more indicative of the real restenotic process inside the stent. Furthermore, in bare metal and sirolimus-eluting stent there is no evidence of edge effect as conversely present with radioactive stents21.
IVUS analysis
Quantitative IVUS analysis was performed by an independent expert not performing any of the procedures and blinded to treatment allocation. The analysis was executed using a validated software (Curad, version 4.32, Wijk bij Duurstede, The Netherlands), allowing semi-automated detection of luminal and stent boundaries in reconstructed longitudinal planes (L-mode views)22. Due to catheter motion during the cardiac cycle, non-gated IVUS pullbacks result in a saw-tooth-appearance of the coronary segment on the longitudinal views and thus interfere with the contour detection algorithms used in IVUS software packages. In order to obtain a smooth appearance of the vessel wall structures in the longitudinal views, the Intelligate image-based gating method was applied23,24. This validated technique eliminates catheter-induced artefacts by retrospectively selecting end diastolic frames, thus resulting in more reliable volumetric measurements. This method has been expanded by introducing the so-called Intelliview, which shows, during the analysis of the end-diastolic L-mode view, the corresponding complete cardiac cycle of the IVUS images made at that particular position on a second computer screen. This enhances the capabilities to determine more accurately the blood-intima-stent interfaces. By detecting the borders of the stent struts and the lumen-intima interface, volumetric quantitative coronary ultrasound analysis was obtained for stent and lumen. The intra- and inter-observer variability of this methodology has been reported25. All the bi-dimensional data were derived from the tri-dimensional reconstruction of the stent-lumen IVUS image, including:
– neointimal hyperplasia index defined as neointimal hyperplasia volume divided by the length of the stent,
– lumen and neointimal hyperplasia cross sectional areas and diameters (minimum, maximum and mean).
As for the QCA analysis, we defined the IVUS position of MLD in the stent as the distance of MLD from the proximal edge of the stent, in this case only at follow-up. In detail, we knew from the software the frame numbers where the stent started and where the MLD was along the IVUS pullback, and we knew the frame thickness (in mm). Thus, “absolute” MLD position was calculated as the difference between the frame where the stent started and the frame where the MLD was located, multiplied for the frame thickness. The “relative” MLD position was calculated as the percentage ratio between the “absolute” MLD position and the stent length computed as the difference between the last and the first frame where the stent was visible (meaning the end and the begin of the stent itself) multiplied for the frame thickness.

Statistical analysis
Data are presented as medians [interquartile ranges] if continuous or as frequencies (percentages) if dichotomous. Comparisons were respectively done with the Mann Whitney U test or the chi-square test. Bivariate correlations were graphically represented with scatter plots and the degree of these correlations was tested with the non-parametric Spearman’s rho correlation coefficient. In case the same variable was measured with different techniques (as it is the case of MLD, measured with QCA and IVUS), a Bland-Altman graph (plotting on the X axis the mean of the values measured with the different techniques and on the Y axis the difference between the same values) was graphically implemented in order to obtain the degree of agreement between the techniques. A 2-sided p-value <0.05 was considered significant.
Results
The baseline characteristics of the patients and lesions enrolled have been already described18,20. Overall, 93 lesions (49 treated with bare metal stent and 44 with sirolimus-eluting stent), from 72 patients, were included in the present QCA analysis because three patients did not undergo angiographic follow-up (Table 1).
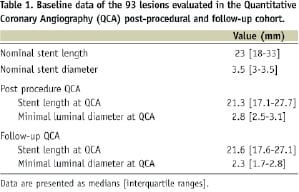
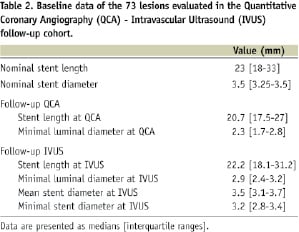
IVUS was performed in 73 lesions (39 treated with bare metal stent and 34 with sirolimus-eluting stent) from 59 patients at 6-month follow-up and this cohort constituted the IVUS-QCA study population (Table 2).
QCA relocation of MLD
On average, the site of MLD shifted from post-procedure to follow-up an “absolute” distance of 5.8 mm [2.5-10.2] and a “relative” value of 29% [10-46], without difference between bare metal and sirolimus-eluting stent (respectively p=0.65 and p=0.44). The “absolute” and “relative” relocations were respectively 5.9 mm [2.4-11.1] and 33% [12-47] after bare metal stent versus 5.1 mm [2.5-8.9] and 26% [10-45] after sirolimus-eluting stent.
In-stent late loss was not correlated to the “absolute” MLD relocation in the overall population (rho= 0.14, p=0.17), in the bare metal stent group (rho= 0.24, p=0.10) and in the sirolimus-eluting stent group (rho= -0.05, p=0.74) (figure 1A). There was also no correlation for the “relative” MLD relocation in the overall population (rho= 0.03, p=0.81), in the bare metal stent group (rho=0.16, p=0.26) and in the sirolimus-eluting stent group (rho=–0.18, p=0.23) (Figure 1B).
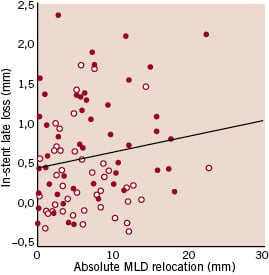
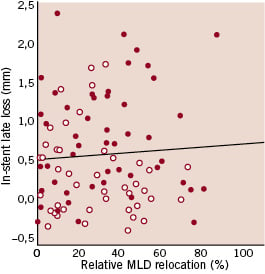
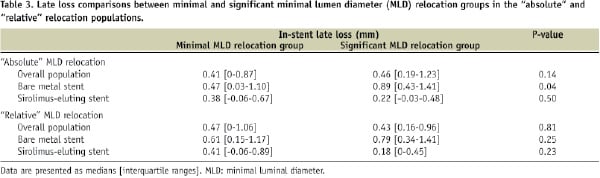
Figure 1. Scatter plot for in-stent late loss versus the “absolute” (A) or the “relative” (B) minimal luminal diameter (MLD) relocation, showing in both cases lack of correlation between the parameters considered (red dots: bare metal stents, white dots: sirolimus-eluting stents).
To further assess the potential impact of MLD relocation on late loss, we divided our study lesions in two groups, namely lesions in which minimal versus significant relocation occurred, according to the median value of “absolute” and “relative” MLD relocation. Of interest, eight out of 46 (17.4%) lesions with minimal (< 5.8 mm) “absolute” MLD relocation had significant (> 29%) “relative” MLD relocation, while nine out of 47 (19.1%) lesions with significant (>5.8 mm) “absolute” MLD relocation had minimal (<29%) “relative” MLD relocation. No differences were noted in late loss value between the minimal and the significant relocation groups according to the “absolute” or to the “relative” value, except in the bare metal stent lesions, in which late loss was slightly significantly higher when MLD shifted >5.8 mm in “absolute” value (Table 3).
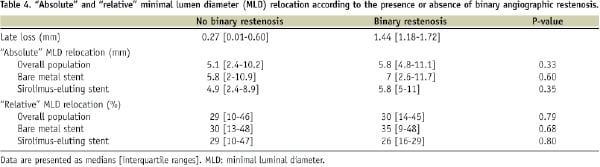
We also performed a second sensitivity analysis, assessing the degree of MLD relocation according to presence or absence of binary restenosis (which is an indicator of higher or lower late loss) at the follow-up coronary angiogram. In the whole cohort considered, there were 20 restenoses out of 93 lesions (21.5%): five out of 44 in the sirolimus eluting stent group and 15 out of 49 in the bare metal stent group. Also in this case, no differences were noted in the degree of relocation according to the presence or absence of binary restenosis (Table 4).
IVUS-QCA correlations
In the subgroup of patients undergoing IVUS at 6-month follow-up, both the “absolute” and “relative” position of the angiographic MLD along the stent at follow-up failed to correlate respectively with the “absolute” and “relative” position of the IVUS in-stent MLD (absolute value: rho=0.01, p=0.91, relative value: rho=0.19, p=0.11). This remained true analysing bare metal or sirolimus-eluting stents separately (Figures 2A and 2B).
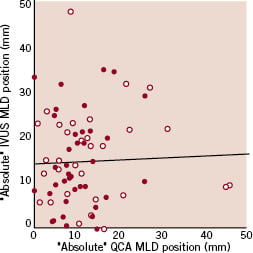
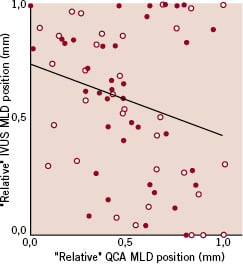
Figure 2. Scatter plot for the “absolute” (A) or “relative” (B) minimal luminal diameter (MLD) position along the stent at follow-up analysed by quantitative coronary angiography (QCA) versus the same value analysed by intravascular ultrasound (IVUS), showing in both cases lack of correlation between the parameters considered (red dots: bare metal stents, white dots: sirolimus-eluting stents).
Angiographic and IVUS in-stent MLD values at follow-up well correlated in the overall population (rho=0.76, p<0.001), and in the two types of stent separately (rho for bare metal stent=0.81, rho for sirolimus-eluting stent=0.61) (Figure 3).
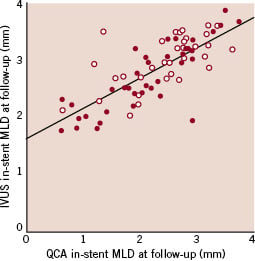
Figure 3. Scatter plot for quantitative coronary angiography (QCA) versus intravascular ultrasound (IVUS) minimal luminal diameter (MLD) values, showing good correlation (rho=0.76, p<0.001) (red dots: bare metal stents, white dots: sirolimus-eluting stents).
However, on average QCA underestimated MLD of 0.55 mm (±0.49 mm) with respect to IVUS and this was more manifest at lower values of MLD as evident from the Bland Altman plot (Figure 4).
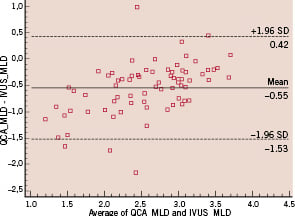
Figure 4. Bland Altman plot for the mean of the quantitative coronary angiography (QCA)-based and intravascular ultrasound (IVUS)-based minimal luminal diameter (MLD) values versus the difference between the same values, showing an average 0.55 mm underestimation of QCA MLD with respect to IVUS MLD, more evident for lower values of MLD.
Overall, the ability of angiographic in-stent late luminal loss to “predict” IVUS parameters of restenosis (maximum neointimal hyperplasia diameter, neointimal hyperplasia index and maximum neointimal hyperplasia area) was moderate with a rho ranging between 0.46 and 0.54 (Figure 5).
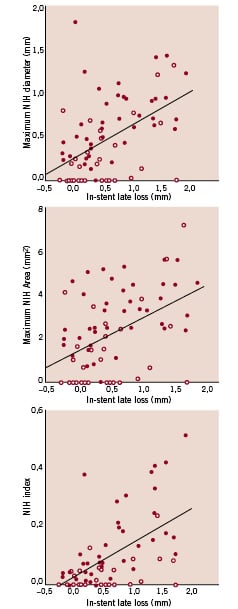
Figure 5. Scatter plot for in-stent late loss versus maximum neointimal hyperplasia (NIH) diameter (A), maximum NIH area (B) and NIH index (defined as NIH volume / stent length) (C), showing overall moderate correlation between late loss and the intravascular ultrasound parameters of restenosis (rho between 0.46 and 0.54) (red dots: bare metal stents, white dots: sirolimus-eluting stents).
Discussion
Although late luminal loss has been extensively applied to investigate the performance of drug-eluting stents3-7, this angiographic parameter has already shown several limitations9-15. Our study offers additional fuel to this debate.
First, in our analysis of stented SVG, angiographic MLD relocation is a frequent phenomenon, as already shown in previous studies focused in native coronary arteries16,17. MLD relocation has the potential to impact adequate calculation of late loss and thus analysis and interpretation of stent trial results. Second, high values of in-stent late loss were measured also when significant in-stent MLD relocation occurred. Conversely to what we anticipated, for elevated values of late loss follow-up MLD was not necessarily located close to the post-procedural MLD position. Indeed, despite post-procedural MLD is a well-established predictor of restenosis19, our data suggest that the restenotic process described by QCA does not necessarily occur at the site of post-procedural MLD but also somewhere else along the stent. Third, we found that QCA and IVUS follow-up MLD values have a good correlation, but also that QCA underestimates MLD mainly for lower values. This finding has been already shown in previous reports10. The novel information of our study is that the MLD position along the stent at follow-up is different when analysed with QCA or IVUS, despite the acceptable correlation of MLD values between the two techniques. This data further reinforce the limitations of QCA in describing the restenotic process.
Late loss is a bi-dimensional parameter defined as the difference between two focal measurements, namely post-procedural and follow-up MLD. These MLD are measured at different time points, thus for one late loss value two measures are exposed to the inherent 0.2 mm resolution limit of QCA (which barely correspond to the facet of one pixel). Moreover, the calibration factor for each MLD measurement is calculated using a different guiding catheter as reference for each time point. All these factors create systematic and random errors, and may explain the poor accuracy and precision of QCA analysis. Of interest, all the aforementioned limitations of QCA appear to remain valid also in SVG, which could potentially constitute a simpler conduit for appropriate QCA analysis, due to the lack of side branches and overlap with other vessels.
The historical value of late loss remains undeniable1. However, with the present study we offer additional limitations of the reliability of this parameter to those already known. In particular, in the drug-eluting stent era, absolute late loss values became far smaller than values in the bare metal stent era. Thus, while late loss can be relatively reliable when comparing bare metal to drug-eluting stents due to the expected large difference between the devices, its value can become trivial in trials comparing different types of drug-eluting stents. Indeed, little late loss differences detected after deployment of different drug-eluting stents can be still significant (due to the lower power needed by continuous endpoints to find statistical significance), but their real angiographic and clinical implication can be negligible. We thus raise doubts about the consistency of late loss as surrogate for the restenotic process mainly when differences between devices are small (in the order of 0.1-0.4 mm). Indeed as the resolution of QCA is approximately 0.2 mm (i.e. around 1 pixel), late loss, as derived by two different QCA measurements, can be even more affected by this resolution limit.
Limitations
The major limitations affecting this study are related to the overall small sample size of the cohort analysed and to the lack of IVUS investigation performed immediately after the deployment of the stent but only at 6-month follow-up control. Furthermore, there are also inherent limitations in the techniques used to generate the measurements. In particular, stent length as measured by QCA and by IVUS can be shorter than the real length due respectively to foreshortening in a non-perpendicular angiographic view and to IVUS catheter forward-backward movement inside the stent. Moreover, the MLD in case of eccentric vessel appearance can be underestimated by a single monoplane QCA analysis. In any case, these two techniques, despite these systematic limitations, are nowadays the most commonly used tools to evaluate the performance of intracoronary devices.
Conclusions
The findings of our study suggest the need for a critical re-evaluation of commonly used QCA endpoints, such as late loss, for upcoming drug-eluting stent trials and the use of more precise techniques to describe with more accuracy the restenotic process.

