Abstract
Aims: We have sometimes encountered difficulty in stent positioning, and managed to achieve optimal positioning of the stent by luck when there was extensive movement of the stent delivery system in association with the cardiac cycle. We assessed the safety and efficacy of rapid ventricular pacing in order to achieve precise positioning of the stent in this percutaneous coronary intervention (PCI) situation.
Methods and results: Among 363 patients who underwent PCI, difficulty in positioning of the stent was encountered in 7 consecutive patients due to extensive movement of the stent delivery system. We applied rapid ventricular pacing in these 7 patients. We measured the length of motion of the stent delivery system relative to the coronary artery and systolic blood pressure before and under rapid ventricular pacing at a rate of 160 min-1. The extent of motion was markedly reduced by rapid ventricular pacing (7.3±2.6 mm to 1.7±0.6 mm; p<0.001). Systolic blood pressure was decreased slightly by rapid ventricular pacing (116±15 mmHg to 90±7 mmHg; p=0.002), but there were no cases of haemodynamic degeneration or ventricular arrhythmia.
Conclusions: Rapid ventricular pacing is a safe and promising option for precise stent positioning, when movement of the stent delivery system prevents precise deployment.
Introduction
Precise positioning of a coronary stent is sometimes essential because of the lesion anatomy during percutaneous coronary intervention (PCI). However, we have occasionally encountered cases in which precise positioning of the stent is prevented by extensive movement of the stent delivery system in association with the cardiac cycle. There are several techniques to reduce cardiac cycle-dependent motion of the stent delivery system, including deep engagement of the guide catheter into the coronary artery, positioning of the stent back and forward, and slow inflation until 1-3 atmospheres to create friction between the stent delivery system and the target vessel wall. However, it sometimes remains difficult to reduce this motion and it is a matter of luck to deploy the stent at the optimal site. Rapid ventricular pacing is an alternative method that can reduce cardiac cycle-dependent heart motion. In addition to attenuating heart motion, it can reduce pulsatile aortic flow. This technique has recently been used for precise positioning of heart valves during percutaneous prosthetic aortic valve implantation1-4. In the present study, we first applied rapid ventricular pacing to achieve precise stent delivery during PCI when: 1) precise positioning of the stent was essential because of the lesion anatomy; and 2) it was prevented by extensive movement of the stent delivery system in association with the cardiac cycle. We assessed the safety and efficacy of rapid ventricular pacing in this PCI situation.
Methods
Study population
A total of 363 patients underwent PCI according to routine clinical indications between April 2006 and October 2006 at our institution. Difficulty was encountered in positioning of the stent in 7 consecutive patients (7 lesions) due to extensive movement of the stent delivery system. We applied rapid ventricular pacing to achieve precise stent delivery in these 7 patients. This study was performed in compliance with the Declaration of Helsinki with regard to investigations in human subjects, and the study protocol was approved by the ethics committee of our hospital. Written informed consent was obtained from all patients before cardiac catheterisation.
Study protocol
Coronary angiography and PCI were performed using the standard femoral or radial approach. All patients received intravenous administration of heparin (100 U/kg). The stent delivery system was placed around the target lesion. A pacing catheter was inserted via the femoral vein and positioned in the apical portion of the right ventricle, and rapid ventricular pacing was then induced at a rate of 100 to 160 min-1 with monitoring of electrocardiography and arterial pressure. After rapid ventricular pacing, we continued ventricular pacing at a rate of 50 min-1 in the case of overdrive suppression by rapid pacing. Using the computerised quantitative coronary angiography analysis system (CRS-PO+, GE Medical Systems, France), we measured the greatest distance of motion of the stent delivery system relative to the coronary artery during the cardiac cycle and systolic blood pressure before and under rapid ventricular pacing at a rate of 160 min-1 over a period of 30 seconds. Finally, we implanted the stent at the optimal site of the coronary artery under rapid ventricular pacing.
Left ventriculography was performed at 30° right anterior oblique projection with contrast medium before the PCI procedures. The global left ventricular ejection fraction (%) was evaluated with the area-length method5.
Statistical analysis
All data are given as means±SD. All statistical analyses were performed using StatView (Abacus Concepts Inc., Berkeley, CA, USA). The extent of motion of the stent delivery system and systolic blood pressure before and after rapid ventricular pacing were compared using the paired Student’s t-test (two group t-test). P < 0.05 was considered to indicate statistical significance.
Results
Representative case
A 51-year-old man with effort angina pectoris underwent PCI to 90% stenosis in the mid-portion of the left anterior descending coronary artery (LAD). We performed coronary angiography via the femoral approach with a guide catheter (6 Fr). Coronary angiography (Figure 1A) and intravascular ultrasound indicated a large difference in reference luminal diameter between the segments proximal and distal to the stenotic lesion.
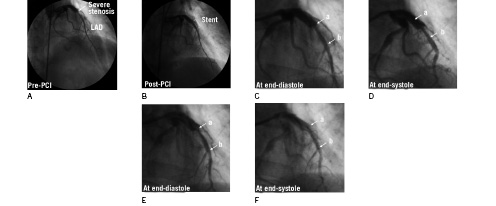
Figure 1. Coronary angiography of the LAD artery in the right anterior oblique cranial projection. A: Baseline angiography showing severe stenosis in the mid-LAD (arrow) and just beyond the proximal end of the stenosis, the luminal diameter sharply increased to 5.2 mm. B: Final angiography showing the ideal positioning for stent implantation. C and D: Long range (7.2 mm) movement of the stent delivery system from end-diastole to end-systole. E and F: The dramatic reduction in movement of the stent delivery system by rapid ventricular pacing at rate of 160 min-1. a = proximal edge of the stent; b = distal edge of the stent. The accompanying moving images can be seen at www.eurointervention.org
The proximal and distal reference luminal diameters were 5.2 mm and 3.3 mm, respectively. The minimal luminal diameter at the site of the stenotic lesion was 1.8 mm and the lesion length was 13 mm. Luminal diameter increased sharply to 5.2 mm just beyond the proximal end of the stenosis. The position of the stent was crucial in this case, and we planned to implant a Cypher® stent measuring 3.5x18 mm (Cordis, Johnson and Johnson Interventional Systems, Warren, NJ, USA) 1 mm proximal to the proximal end of the stenotic lesion to minimise the length of stent placement at the large proximal reference lumen (5.2 mm) because the maximum dilation diameter of the 3.5 mm Cypher® stent is around 5.0 mm and the stent struts would not be fully attached to the vascular inner surface at the large proximal reference lumen. We attempted direct stenting, but the stent delivery system moved extensively (7.2 mm) in association with the cardiac cycle (Figure 1C and 1D). The greatest distance coincided with the length of motion from end-systole to end-diastole. We changed the position of the guide catheter, guidewire and stent delivery system to reduce cardiac cycle-dependent motion of the stent delivery system. However, these adjustments did not reduce the movement. Then, we positioned a pacing catheter in the apical portion of the right ventricle and rapid ventricular pacing markedly reduced the movement of the stent delivery system. When a pacing rate of 160 min-1 was achieved, motion of the stent delivery system relative to the LAD disappeared almost completely (Figure 1E and 1F). Systolic blood pressure decreased to around 90 mmHg (Figure 2).
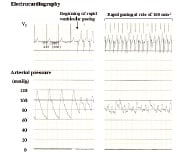
Figure 2. Electrocardiography and arterial pressure monitoring. Rapid ventricular pacing reduced systolic blood pressure from 120 to around 90 mmHg, but did not cause haemodynamic corruption.
We successfully implanted the stent 1 mm proximal to the proximal end of the stenotic lesion (Figure 1B). Post-dilatation was performed with a 5.0x10 mm balloon catheter to dilate only the proximal stent site also under rapid ventricular pacing. Intravascular ultrasound showed that the stent was dilated to 5.2 mm proximal to the lesion and the stent struts were completely attached to the reference lumen.
Patient characteristics
Table 1 summarises the patient and lesion characteristics.
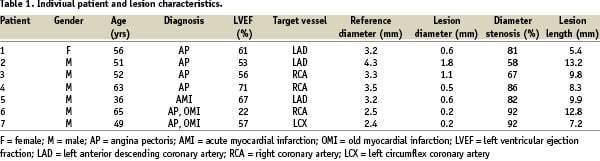
The reasons for precise positioning of the stent were as follows: 1) ostial lesion (patients #1 and 7); 2) bifurcated lesions (patients #3, 5 and 6); 3) large difference in reference luminal diameter between the segments proximal and distal to the stenotic lesion as in the representative case (patient #2); or 4) mild stenosis in the reference lesion, which should be avoided by deployment of stent edge (patient #4).
Safety and efficacy of rapid ventricular pacing
The extent of motion of the stent delivery system relative to the coronary artery was markedly reduced by rapid ventricular pacing at a rate of 160 min-1 (Figure 3A). Systolic blood pressure was decreased by rapid ventricular pacing at a rate of 160 min-1 (Figure 3B), but there was no haemodynamic degeneration or ventricular arrhythmia, and blood pressure recovered rapidly to the baseline level after pacing was stopped.
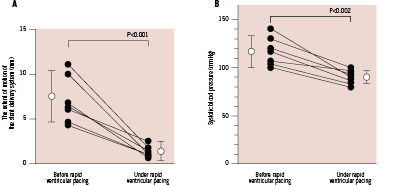
Figure 3. The extent of motion of the stent delivery system relative to the coronary artery and systolic blood pressure before and under rapid ventricular pacing at a rate of 160 min-1. A: The extent of motion of the stent delivery system was markedly reduced by rapid ventricular pacing (7.3±2.6 mm to 1.7±0.6 mm; p<0.001). B: Systolic blood pressure was decreased slightly by rapid ventricular pacing (116±15 mmHg to 90±7 mmHg; p=0.002).
Discussion
This is the first report of the usefulness of rapid ventricular pacing for stent deployment at the optimal site. Right ventricular pacing can reduce movement of the stent delivery system in the coronary artery in association with the cardiac cycle, and facilitate stent implantation in the ideal position during PCI. This technique is useful when: 1) precise positioning of the stent is essential; and 2) this is prevented by extensive movement of the stent delivery system in association with cardiac cycle. In clinical practice, we have encountered such situations in around 2% of PCI procedures such as seen in the present study, and managed to achieve optimal positioning of the stent only by luck. With minimal effort of rapid ventricular pacing, we can avoid the risk of mis-positioning of the stent.
Precise positioning of devices for the target lesions is required for therapeutic catheter procedures for congenital heart diseases, transluminal thoracic aortic surgery and transluminal aortic stent graft implantation. Adenosine-induced ventricular standstill6-9, transient induction of ventricular fibrillation10 and balloon occlusion of the vena cava have been used for this purpose. However, these methods lead to transient haemodynamic degeneration and cannot be continued for long periods. In the present study population, we performed 30-second rapid ventricular pacing at 160 min-1 and systolic blood pressure decreased transiently to around 80-90 mmHg without haemodynamic degeneration, ventricular arrhythmia or angina. Blood pressure then recovered rapidly to the previous value after pacing. However, careful monitoring of symptoms, electrocardiography and arterial pressure is required to avoid these adverse events. In the present study population, the average left ventricular ejection fraction was 55%, and this good contractile function may explain the preserved blood pressure during rapid ventricular pacing. If rapid ventricular pacing is performed in patients with reduced cardiac function, sufficient attention must be given to haemodynamic changes.
Conclusions
Although its application is limited, rapid ventricular pacing is a safe and promising option for precise stent positioning when this is prevented by extensive movement of the stent delivery system in association with cardiac cycle.
Online data supplement
video 1. Pre-rapid ventricular pacing (Figure 1C and 1D).
video 2. Under rapid ventricular pacing (Figure 1E and 1F).

