
Abstract
Aims: This study aimed to compare the clinical outcomes of patients undergoing transfemoral transcatheter aortic valve implantation (TAVI) via a percutaneous or surgical cut-down approach.
Methods and results: Between October 2013 and July 2015, 586 patients underwent transfemoral TAVI according to the Optimized CathEter vAlvular iNtervention (OCEAN)-TAVI registry (percutaneous approach, n=305; surgical cut-down approach, n=281). After propensity matching, 166 patients underwent transfemoral TAVI via each approach. Major vascular complications, as defined per the Valve Academic Research Consortium-2 criteria, were found less frequently in patients who underwent a percutaneous approach (15.1% vs. 27.1%, p<0.01), and femoral artery injuries requiring surgical repair were mostly the result of a closure device failure (seven cases, 4.2%). In these patients, major bleeding was less (7.2% vs. 16.9%, p=0.01) and blood transfusion less frequent (21.1% vs. 38.0%, p<0.01); therefore, cases of acute kidney injury (AKI) were rare (6.0% vs. 15.1%, p<0.01).
Conclusions: Transfemoral TAVI using the percutaneous approach proved safe and feasible and resulted in fewer major vascular complications, bleeding and AKI events compared to the surgical cut-down approach.
Abbreviations
AKI: acute kidney injury
ICU: intensive care unit
IQR: interquartile range
MSCT: multislice computed tomography
OCEAN: Optimized CathEter vAlvular iNtervention
VARC-2: Valve Academic Research Consortium-2
Introduction
Transcatheter aortic valve implantation (TAVI) has become an essential part of everyday practice in the treatment of patients with severe symptomatic aortic stenosis who are ineligible or otherwise considered to be at high risk of complications for conventional surgical aortic valve replacement1-3. Various access routes have been described in TAVI procedures4-7. First-generation transfemoral TAVI devices required larger calibre sheaths with 22 or 24 Fr inner diameters, requiring the use of surgical cut-down and closure to ensure appropriate access and haemostasis. However, the surgical approach is associated with wound complications that may delay the early mobilisation of aged patients8. Recently, sheath diameters have decreased. Despite the sheath diameter reduction, vascular complications, as defined under the Valve Academic Research Consortium-2 (VARC-2) criteria9, remain a serious concern associated with the transfemoral TAVI procedure. The percutaneous approach for femoral access in transfemoral TAVI is a powerful alternative to the surgical approach and has become mainstream with reduced sheath diameters. However, little is known about the advantages and disadvantages of this percutaneous approach in patients with limited iliofemoral access, such as our cohort. The aim of this study was to compare the percutaneous and surgical cut-down approaches with regard to the clinical outcomes of patients undergoing transfemoral TAVI.
Methods
PATIENT POPULATION
The Optimized CathEter vAlvular iNtervention (OCEAN)-TAVI registry is a Japanese multicentre prospective registry. Between October 2013 and July 2015, 749 patients in this registry with symptomatic severe aortic stenosis underwent TAVI using the Edwards SAPIEN XT prosthesis (Edwards Lifesciences, Irvine, CA, USA) via the transfemoral or transapical approach. All surgeons used 16/18/20 Fr expandable eSheaths (Edwards Lifesciences) with valve sizes of 20/23/26/29 mm. Among the 749 patients, 586 (78.2%) underwent TAVI via the transfemoral access. Among those treated via the transfemoral approach, 305 (52.0%) underwent percutaneous femoral artery access using the Perclose ProGlide® suture-mediated closure system (Abbott Vascular, Santa Clara, CA, USA). In contrast, 281 (48.0%) were accessed via surgical cut-down and closure. In all of the institutions, the surgical cut-down approach was performed by a cardiac or vascular surgeon. The choice of closure method for femoral arterial access was determined at the discretion of each centre after discussion by the surgical team. All institutions used both approaches. The percutaneous or surgical approach was chosen by each operator according to vessel calcification, tortuosity, and vessel size. Furthermore, the vascular access strategy was always determined prior to the procedure.
VESSEL CHARACTERISATION AND DEFINITIONS
The minimal luminal diameters of the iliofemoral arteries on the vascular access side were measured using multislice computed tomography (MSCT). Vessel tortuosity and calcifications were evaluated using MSCT as previously described10,11. Tortuosity was graded as none, mild (30°-60°), moderate (60°-90°), or severe (>90°). MSCT calcification was graded as none, mild (some calcification), moderate (the arterial course can be seen without contrast dye injection), or severe (heavily calcified iliofemoral arteries)12,13. The sheath-to-femoral artery ratio was defined as the ratio of the sheath’s outer diameter to the femoral artery’s minimal luminal diameter3, which was calculated for all patients.
VASCULAR COMPLICATION DEFINITIONS
Major vascular complications included any aortic dissection, aortic rupture, annulus rupture, left ventricle perforation, access-site, or access-related vascular injuries leading to death or life-threatening bleeding, distal embolisation, unplanned intervention, new ischaemia, and nerve injury. Complications not meeting these criteria were identified as minor vascular complications. The management of vascular complications was left to the physician’s discretion.
BLEEDING AND DEFINITIONS
Definitions of bleeding were also framed in accordance with the VARC-2 criteria. Life-threatening bleeding is defined as that causing hypovolemic shock or severe hypotension requiring vasopressors or surgery, an overt source of bleeding with a decrease in haemoglobin levels >5 g/dL or requiring a red blood cell transfusion of >4 units. Major bleeding is defined as a decrease in haemoglobin levels >3 g/dL or requiring a transfusion of two or three units of whole blood. Minor bleeding is defined as any bleeding worthy of clinical mention that does not qualify as life-threatening or major.
ACUTE KIDNEY INJURY AND DEFINITIONS
Acute kidney injury (AKI) was divided into stages 1-3 in accordance with the VARC-2 criteria. Stage 3 is an increase in serum creatinine to >300%, serum creatinine of >4.0 mg/dL with an acute increase ≥0.5 mg/dL, urine output <0.3 mL/kg/hr for 24 hours or anuria for >12 hours. Stage 2 is an increase in serum creatinine to 200-299%, urine output <0.5 mL/kg/hr for >12 hours but <24 hours. Stage 1 is an increase in serum creatinine to 150-199%, urine output <0.5 mL/kg/hr for >6 hours but <12 hours.
STATISTICAL ANALYSIS
Quantitative variables were assessed for normal distribution with the Shapiro-Wilk test and expressed as median and interquartile range (interquartile range [IQR], 25-75%). Qualitative variables are expressed as numeric values and percentages. A comparison of quantitative variables was performed using the Mann-Whitney U test. The chi-square test or Fisher’s exact test was used to compare qualitative variables. The propensity score was calculated using a non-parsimonious multivariate logistic regression model. We performed rigorous adjustment for significant differences in the baseline clinical characteristics and procedural variables of patients with propensity score matching using the following algorithm: nearest neighbour matching with a calliper width of 0.1 standard deviation of the propensity score and no replacement. The propensity score comprised the variables sex, age at time of TAVI, body surface area, chronic kidney disease >stage 2 (estimated glomerular filtration rate <60 mL/min/1.73 m2), aortic valve area, femoral artery diameter, external iliac diameter, common iliac diameter, minimal lumen diameter of the iliofemoral artery, greater than mild calcification degree, greater than mild tortuosity degree, outer sheath diameter, eSheath diameter, valve size, and left ventricular ejection fraction <40%. After matching, continuous variables were compared using the Wilcoxon signed-rank test. Differences for matched categorical variables were analysed with McNemar’s test. Statistical significance was defined as values of p<0.05. All data were processed using SPSS statistical software, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT CHARACTERISTICS AND PROCEDURAL VARIABLES
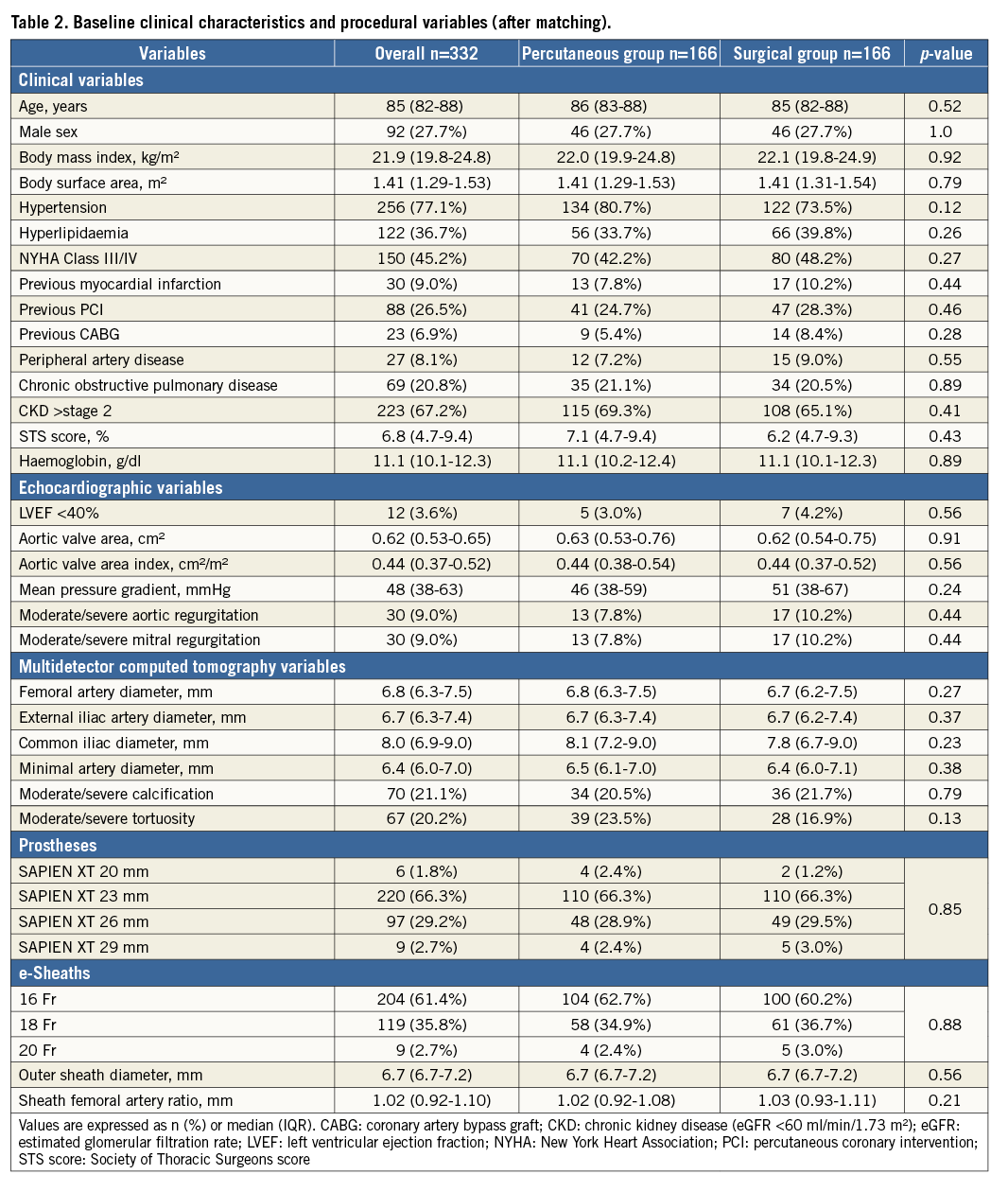
Baseline clinical and procedural characteristics of the two study groups are presented in Table 1. In this study, no case converted from the percutaneous to the surgical approach. Propensity score matching based on baseline characteristics and anatomic and procedural data resulted in 166 patients who underwent the percutaneous approach: this cohort was identified as the “percutaneous group”. These patients were matched with 166 patients who underwent the surgical cut-down approach, those comprising the “surgical group” (Figure 1). Propensity score matching revealed that the groups were well matched with no significant differences in baseline characteristics or procedural variables (Table 2). The Hosmer-Lemeshow goodness-of-fit test for this model had a p-value of 0.95, and we calculated an area under the receiver operating characteristic curve of 0.67.
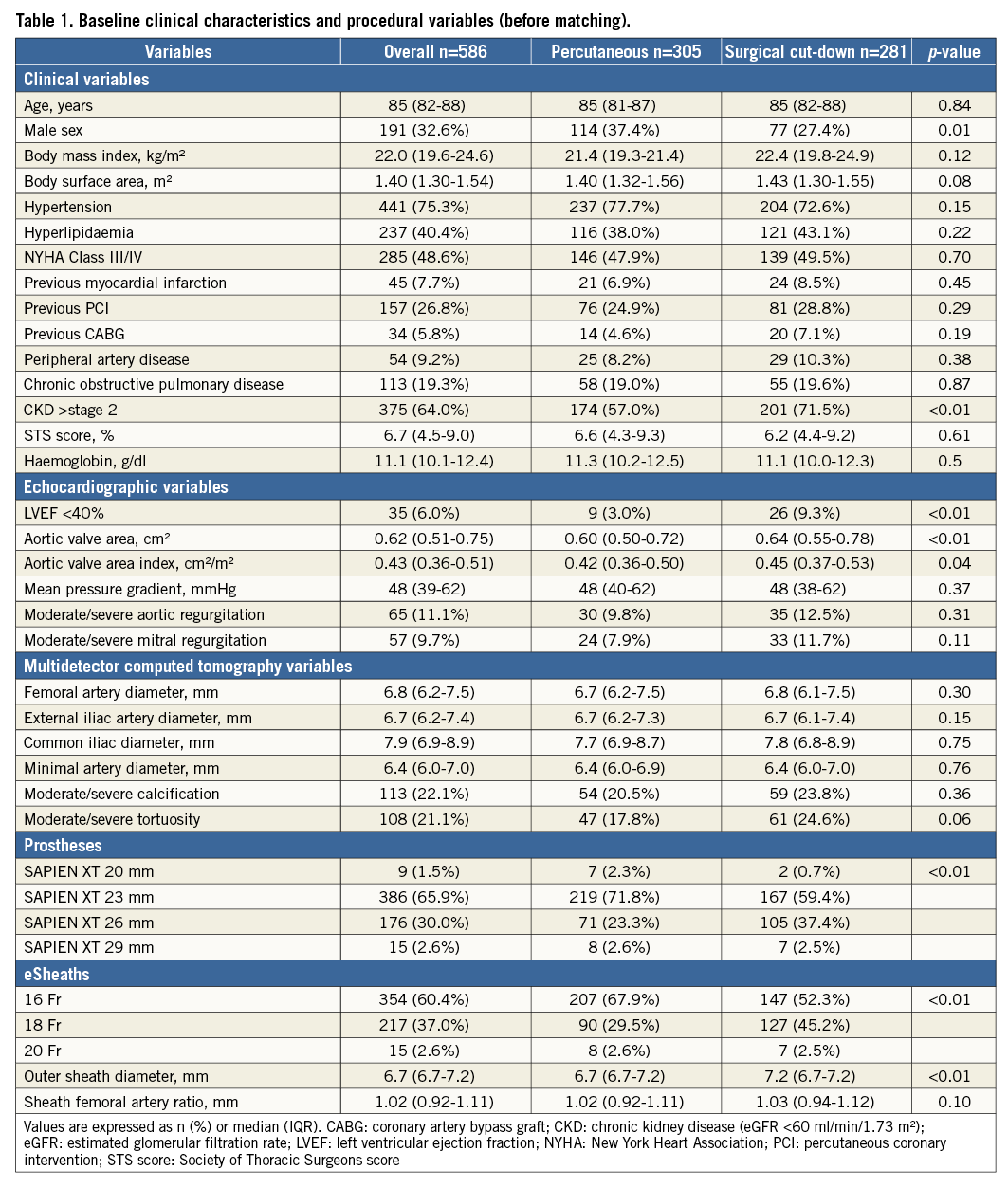
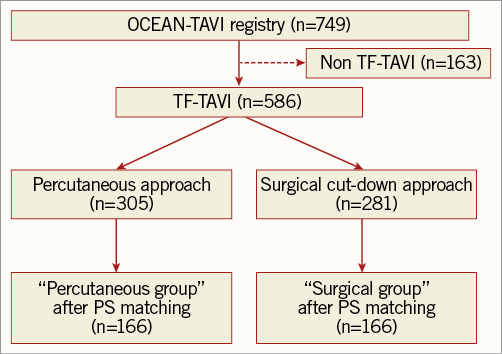
Figure 1. Study population. OCEAN: Optimized CathEter vAlvular iNtervention; PS: propensity score; TAVI: transcatheter aortic valve implantation; TF: transfemoral
PROCEDURAL AND IN-HOSPITAL OUTCOMES AND COMPLICATIONS
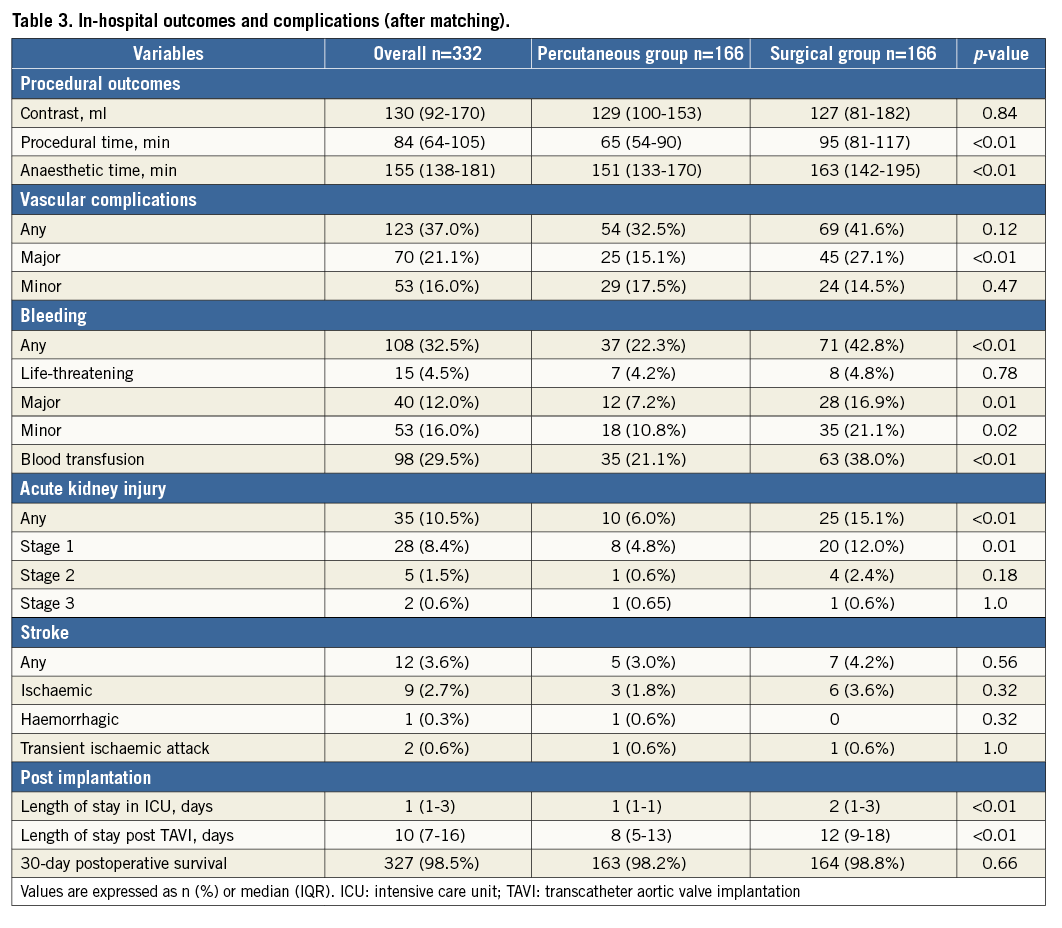
Procedural and in-hospital outcomes and complications are summarised in Table 3 after propensity score matching. Procedural and anaesthesia times were significantly shorter for the percutaneous group. The incidence of major vascular complications as per the VARC-2 criteria was more rarely encountered in the percutaneous group (15.1% vs. 27.1%, p<0.01). Complications of major and minor bleeding per the VARC-2 criteria were more rarely seen in the percutaneous group (7.2% vs. 16.9%, p=0.01; 10.8% vs. 21.1%, p=0.02, respectively). Fewer transfusions were required in the percutaneous group (21.1% vs. 38.0%, p<0.01). Cases of AKI were significantly rare among the percutaneous group (6.0% vs. 15.1%, p<0.01). Length of stay in the intensive care unit (ICU) and hospitalisation duration following TAVI were shorter in the percutaneous group.
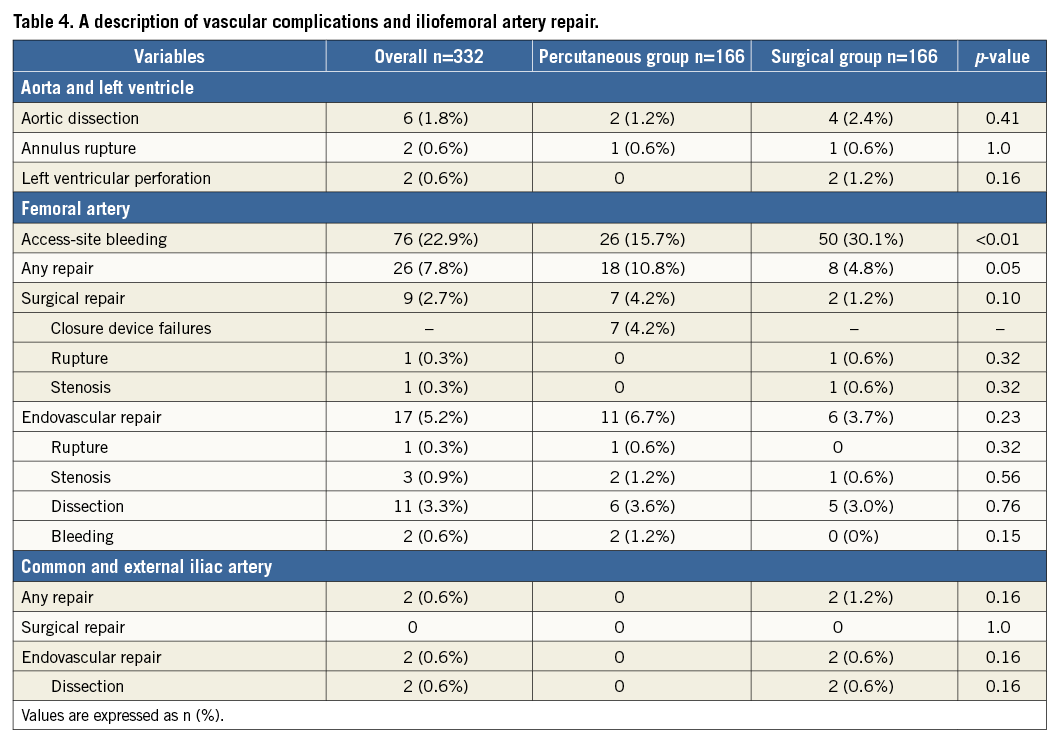
ACCESS-RELATED VASCULAR COMPLICATIONS AND REPAIR
A description of vascular complications and iliofemoral artery repair performed after propensity score matching is detailed in Table 4. Access-site bleeding was significantly rare (15.7% vs. 30.1%, p<0.01), and femoral artery injuries requiring surgical repair were mostly the result of closure device failure (seven cases, 4.2%).
Discussion
This study compared the efficacy and safety of transfemoral TAVI using the percutaneous or surgical cut-down approach. Transfemoral TAVI using the percutaneous approach has been proven safe and feasible, resulting in similar acute and subacute outcomes compared to those seen with the surgical cut-down approach in this study. The percutaneous approach can be the preferred method of choice, especially for elderly patients requiring shorter procedural time, short anaesthetic time, and shorter length of ICU stay. The present study demonstrates that routine application of the percutaneous approach can be advantageous in patients undergoing transfemoral TAVI.
In the transfemoral TAVI procedure, the vascular complications per the VARC-2 criteria present a serious problem for patients. Previous studies demonstrated that the frequency of vascular complications was lower using the surgical cut-down approach14 or a comparable method15. Complications of the iliac artery are more likely to cause haemodynamic failure; however, the incidence of complications in the iliac artery was extremely low in this study. On the other hand, the majority of vascular complications observed in the surgical cut-down approach consisted of bleeding from the wound. Moreover, in the percutaneous approach, surgical repairs primarily consisted of closure device failure. We think that closure device failure remains a serious concern associated with percutaneous approach TAVI and estimate that these complications are due to extended procedure time. Procedural duration and time under anaesthesia were shorter using percutaneous access in this study. Notably, shortening the time under general anaesthesia is considered to support a good prognosis, especially among elderly patients. The percutaneous approach is easily performed under local anaesthesia due to its less invasive nature, whereas the surgical cut-down approach is difficult to perform under local anaesthesia. Moreover, the percutaneous group experienced shorter ICU and hospital stay lengths after transfemoral TAVI, which is consistent with the results from previous studies15,16. Additionally, prolonging the length of stay impairs long-term survival17. Shortening the length of stay particularly benefits aged patients, since it may prevent the exacerbation of dementia and decline in the performance of daily activities.
Concerning the incidence of bleeding complications, a previous study demonstrated that minor bleeding as per the VARC-2 criteria was more often seen with the surgical cut-down approach than with the percutaneous approach18. A recent study found that vascular closure devices for common femoral artery haemostasis were rarely associated with major bleeding in transfemoral TAVI19. This study reported that bleeding events and blood transfusion interventions occurred more frequently in cases of more severe AKI. Furthermore, another previous study demonstrated that contrast volume was lower in the surgical group since there was less use of contrast, which is usually used to obtain access in the percutaneous approach20. However, the bleeding rate complications were high in the surgical cut-down approach in this study. The total amount of contrast volume used did not differ between the two groups since it was used to check bleeding from the access site in every case. The volume of contrast media associated with increased risk for AKI in elderly TAVI populations was recently determined21. In the present study, the occurrence of AKI-related events was lower in the percutaneous group regardless of pre-procedural kidney function and the contrast media volume used in the TAVI procedure. To our knowledge, no study to date has found a significant difference in the frequency of AKI between the percutaneous and surgical cut-down approaches. However, this result may suggest that AKI is related to the difference in the incidence of bleeding complications and the number of blood transfusions required. We already reported that an increased incidence of AKI is associated with increased mortality in TAVI21. Therefore, this percutaneous approach with less bleeding and reduced transfusions required might have the potential to reduce mortality in this elderly cohort.
Limitations
The present study has several limitations. First, it was conducted using a prospective multicentre TAVI cohort with a relatively small number of patients using a non-randomised design. Second, the inclusion of nine different institutions might have resulted in some bias from differences among clinical treatment procedures. Third, selection bias should be considered with this non-randomised study, even after propensity matching. Furthermore, all cases of the TAVI procedure were performed using the Edwards SAPIEN XT prosthesis in this study. However, the percutaneous transfemoral TAVI approach has resulted in similar outcomes to those of the surgical cut-down approach. Hence, we predict that reducing the sheath diameter using a SAPIEN 3 prosthesis will decrease minor vascular complications using the VARC-2 criteria and that the percutaneous transfemoral TAVI approach may become the main approach. Finally, further studies using larger groups of patients will be required to confirm our results.
Conclusion
The performance of the percutaneous transfemoral TAVI approach proved safe and feasible and resulted in fewer events related to major vascular complications, bleeding, and AKI than the surgical cut-down approach. This study demonstrated that the routine application of the percutaneous approach might reduce acute complications in patients undergoing transfemoral TAVI.
| Impact on daily practice The incidence of major vascular complications was low using the percutaneous approach. Thus, the surgical repairs were mostly required due to closure device failure. Transfemoral TAVI with the percutaneous approach resulted in fewer occurrences of bleeding and AKI than the surgical cut-down approach. To our knowledge, no previous study has found a significant difference in the frequency of AKI between the two approaches; thus, the percutaneous approach, with a lower incidence of bleeding and resultant transfusions, might reduce the incidence of AKI after the TAVI procedure. |
Acknowledgements
The authors thank the investigators and institutions participating in the OCEAN-TAVI registry.
Conflict of interest statement
Y. Watanabe, M. Yamamoto, M. Araki, N. Tada, S. Shirai, and K. Hayashida are proctors for transfemoral TAVI for Edwards Lifesciences. The other authors have no conflicts of interest to declare.

