Abstract
Aims: Despite encouraging results with drug-eluting stents (DES) reported in diabetic patients, the long-term safety is unknown because of very late stent thrombosis (VLST). We investigated the incidence, risk factors and clinical manifestations of VLST in diabetic patients treated with DES, during long-term clinical follow-up.
Methods and results: A total of 610 consecutive diabetic patients underwent PCI with DES. Dual antiplatelet treatment (APLT) for 12 months received 93%, more than 12 months 72% and statin treatment 93% of patients. Clinical follow-up of at least 12 months post DES implantation was obtained in 597/610 (98%) patients. The incidence of VLST was 1.8%, and 1.7% of patients developed stent thrombosis (ST) up to 12 months. All patients with VLST presented with sudden cardiac death and 82% were on dual APLT at the time of the event. In a multivariate model the only predictor for VLST (HR: 20.58, 95% CI 5.17-81.90, p<0.001) and overall ST (HR: 4.38, 95% CI 1.73-11.10, p=0.002) was ejection fraction < 40%.
Conclusions: The incidence of ST in diabetic patients undergoing PCI with DES and receiving dual APLT is low at long-term clinical follow-up. The only predictor for VLST and overall ST was depressed left ventricular systolic function.
Introduction
Patients with diabetes mellitus (DM) constitute a high-risk group and have a higher incidence of cardiovascular disease, complications and mortality, as compared to non-diabetics and furthermore, patients requiring insulin for the treatment of diabetes are more susceptible to adverse cardiac events1,2. The distinct characteristics of coronary artery disease in these patients, such as more diffuse and accelerated with longer lesion lengths and smaller vessel size, make the interventional treatment a real therapeutic challenge.3,4 Patients with DM have worse outcomes than patients without diabetes, irrespective of the invasive therapy they receive, whether it be percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).5,6 Unfortunately PCI with bare metal stents (BMS) in these particular patients is associated with elevated rates of restenosis.7,8 Meanwhile, drug-eluting stents (DES) have brought a revolution in the management of patients with coronary artery disease by reducing restenosis rate in the general population and in diabetic patients as well.3,9-11 The initial enthusiasm associated with the significant positive effect on repeat revascularisation of DES has been limited by safety concerns related to a risk for stent thrombosis (ST), a catastrophic, but rather infrequent complication that results in abrupt coronary artery obstruction, leading very often to myocardial infarction (MI) and/or sudden cardiac death (SCD).12-14 The incidence of this problem does not seem to exceed that seen with BMS for the first year of follow-up,15-17 but concerns have been raised about an increased risk of very late (>1 year) stent thrombosis (VLST) following DES implantation, a different pattern of risk than what had been seen for BMS.18,19
As the efficacy and safety of DES especially in diabetic patients was controversial, we conducted this study in order to assess the incidence and predictors of VLST and overall ST in this high-risk population.
Methods
Between June 20, 2002, and Dec 31, 2005, a total of 610 consecutive diabetic patients underwent PCI in our department, with implantation of at least one DES in their native coronary arteries or aorto-coronary bypass grafts.
Eligible patients had a history of stable or unstable coronary artery disease (stable angina, acute coronary syndromes, including acute myocardial infarction), or silent ischaemia proven by stress test, and a manifest DM, proven by fasting glucose >126 mg/dl or oral glucose challenge >200 mg/dl after two hours, or DM treated with oral antidiabetic agents, insulin or both. PCI was the treatment of choice according to the attending cardiologists and de novo or restenotic lesions was targeted for treatment. Major exclusion criteria were haemorrhagic diathesis, contraindications to the use of aspirin and thienopyridines, coexisting conditions that limited life expectancy to less than 12 months, and lesions within an unprotected left main, except the need for emergency treatment (dissection during PCI).
Coronary stent procedure
Coronary intervention was performed according to standard techniques, including mandated balloon dilation before placement of the stent. Sirolimus-eluting stents were implanted in 487 (80%), paclitaxel-eluting stents in 64 (10%), zotarolimus-eluting stents in 12 (2%) and a combination of DES in 47 (8%) patients. One or more stents were used in order to cover the entire diseased segment; 792 (83%) lesions were treated with sirolimus-eluting, 134 (14%) with paclitaxel-eluting and 28 (3%) with zotarolimus-eluting stents. The use of glycoprotein IIb/IIIa inhibitors was left at the discretion of the interventional cardiologist. Before the index procedure, all patients received oral aspirin (at least 100 mg daily) and oral clopidogrel (a loading dose of 300 to 600 mg as soon as possible during the procedure and then 75 mg daily), and continued dual antiplatelet treatment (APLT) for at least 12 months; the use of dual APLT for a longer period was encouraged and left to the physician’s discretion. Aspirin, or alternately clopidogrel, was continued indefinitely. Patients, who were pretreated with clopidogrel (75 mg once daily) for at least four days, did not receive a clopidogrel-loading dose. Angiographic success was defined as the achievement of residual in-segment stenosis of <20%, associated with TIMI 3 flow in the absence of a dissection. Clinical success was defined as the angiographic success without the occurrence of death, Q-wave myocardial infarction, or repeat target lesion revascularisation (TLR) during hospitalisation.
Follow-up, endpoints and definitions
Clinical outcomes during follow-up were obtained by serial telephone interviews by research fellows. Patients without completion of at least 12 months of follow-up were excluded. Late major clinical events, such as death, non-fatal MI, cerebrovascular accident (CVA), any revascularisation (PCI or CABG) for TLR and non-TLR (due to disease progression), were adjudicated by accompanying source documentation. The primary endpoint was the occurrence of ST (definite, probable, or possible according to the ARC definitions20). Definite ST was determined by angiographic confirmation plus any new ischaemic symptoms, new ischaemic ECG changes, positive cardiac biomarkers within 48 hours, or pathologic documentation at autopsy or in tissue from thrombectomy. Probable ST was considered to be any unexplained death within the first 30 days from stent implantation or any MI related to territory of the implanted stent. Possible ST was considered to be any unexplained death after 30 days following intracoronary stenting. ST was defined as acute, early, late and very late if the event occurred within the first 24 hours, 30 days, >1 month to 1 year or >1 year respectively. Secondary endpoints included the occurrence of major adverse cardiovascular events (MACE) defined as the composite of death, MI, and CVA; CABG, TLR and non-TLR were also considered as secondary endpoints. All deaths were considered cardiac unless a noncardiac cause could be established. TLR was defined as a repeat intervention for a luminal restenosis >50% of the target lesion (ranging from 5 mm proximal to 5 mm distal to the stent), and non-TLR as an intervention for another lesion due to disease progression. MI was defined as the development of new pathological Q waves in at least two contiguous leads, irrespective of the presence of typical symptoms, with an elevated creatine kinase MB fraction level or, in the absence of pathological Q waves, an elevation in creatine kinase MB fraction >3 times the upper limit of normal value.
Statistical analysis
Continuous variables are expressed as mean±standard deviation and categorical variables as percentages. Comparisons between continuous variables were performed using Student’s t-test. Normality of the variables was tested using the Shapiro-Wilk test. Pearson’s chi-square or Fisher exact tests were applied to test associations between categorical variables. Event rates were calculated using the observed person/time in months. Survival analysis was performed using the Cox proportional hazards model with VLST, overall ST, death, or any MACE (death, non-fatal MI, CVA) as end points and the following variable as predictors: gender, age, insulin-dependent DM, PCI indication (stable vs. unstable coronary artery disease), EF<40%, multivessel disease, and previous MI. All statistical analyses were performed with the use of SPSS software (version 12.0, SPSS Inc., Chicago, IL, USA). Statistical significance was considered at a p <0.05 (for two-tailed hypotheses).
Results
Baseline and procedural characteristics
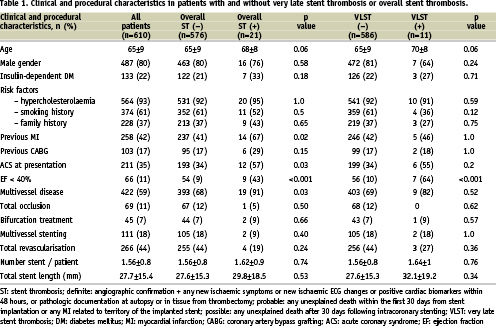
Baseline and procedural characteristics of our study patients as a total and according to the occurrence of VLST or overall ST at follow-up are presented in Table 1. There was a trend for advanced age in patients presented VLST and overall ST (both p=0.06). A significant higher incidence of patients with ejection fraction < 40% was present in VLST and overall ST group (p<0.001); a higher incidence of multivessel disease (p=0.03), history of MI (p=0.02) and acute coronary syndrome as the indication for PCI, p=0.03) was observed in patients presented overall ST.
In-hospital results
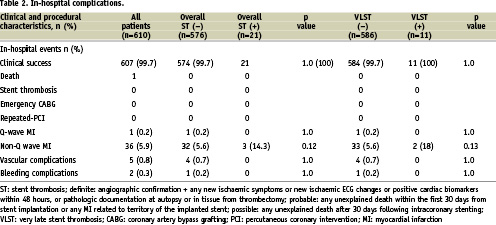
Clinical success was achieved in 607/610 (99.5%) patients (Table 2). One patient died two days after PCI from advanced heart failure. One (0.2%) and 36 (5.9%) periprocedural Q and non-Q myocardial infarctions were observed respectively. There was no ST, CABG or repeat PCI before hospital discharge.
Clinical follow-up
Follow-up (>12 months) was obtained in 597/610 patients (98%). The median follow-up time was 29 months (IQR, 21 to 40 months). Dual APLT for 12 months received 93%, and >12 months 72% of patients; statin treatment at the time of follow-up was receiving 93% of them. The cumulative incidence of death was 5% (cardiac death 3.2%), non-fatal MI 1.7%, any MACE 8.2%, TLR 4.5%, CABG 1.8%, and any revascularisation (TLR and non-TLR due to disease progression) 16.8%. Survival analysis revealed that predictors for death were advanced age (p=0.002), and low ejection fraction (p<0.001), (Table 3). Moreover, for MACE, significant predictors were advanced age (p=0.01) and low ejection fraction (p=0.03). Event-free survivals from death/non-fatal MI, and MACE according to ejection fraction are shown in Figures 1A and B.
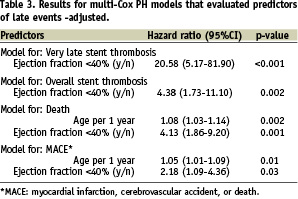
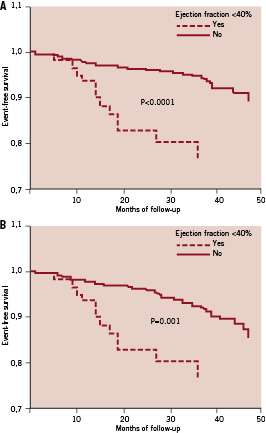
Figure 1. A: Event free-survival from death and/or myocardial infarction according to ejection fraction; B: Event free-survival from death, non-fatal myocardial infarction and cerebrovascular accident according to ejection fraction.
Stent thrombosis
According to the newly defined Academic Research Consortium definitions overall ST occurred in 21 patients (3.5%) (Table 4). Depending on the time of onset, there were 10 events <12 months (median 6 months, IQR 4 to 9 months); four cases presented as acute MI and six as SCD; all patient were on dual APLT at the time of the event. Three cases were confirmed as definite by coronary angiography, two cases corresponded to “probable” (one SCD 30 days after the index procedure and one MI in the territory of the implanted stent), and five cases to “possible”, leading to SCD. VLST occurred in 11 (1.8%) patients, at a median time of 19 months (IQR 14 to 27 months); all patients presented with SCD. No significant differences in the incidence of ST observed between the three types of DES that had been used; from those patients treated with sirolimus-eluting stents, 16 (3%) presented with ST, while four patients (4%) from the paclitaxel- eluting group and one patient (5%) from zotarolimus-eluting group had this complication, (p=ns). Nine out of 11 patients (82%) were on dual APLT at the time of the event. Predictors for VLST and overall ST are reported in Table 3. As it can be seen, low ejection fraction was highly associated with increased risk for overall, as well as VLST, after taking into account various potential confounders mentioned above. The event free survival from overall ST according to ejection fraction is shown in Figure 2.
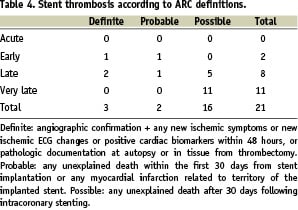
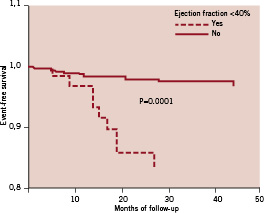
Figure 2: Event free-survival from overall stent thrombosis according to ejection fraction.
Discussion
In the present study, in patients with DM undergoing PCI using DES and receiving long-term dual APLT (up to 12 months 93% and >12 months 72% of patients), the incidence of overall ST was 3.5% during a follow up period up to 4.5 years; in 52% of cases occurred after 12 months (VLST), 82% of patients were on dual APLT at the time of the event, and all presented with SCD. Predictor for VLST, as well as overall ST was depressed ejection fraction. The total incidence of MACE during this long period of follow up was 8.2%, and predictors were age, and low ejection fraction.
Stent thrombosis and DES
Late and VLST is a major concern after DES implantation.14,19 In the present study, overall ST occurred with an incidence of 3.5% at long-term clinical follow-up; the incidence of this complication was 1.7% during the first year of follow-up, and only 0.67% of patients had angiographically documented ST during the entire period of follow-up. Angiographically proven LST observed in 0.5% of patients. Daemen et al12 recently reported a cumulative incidence of angiographically proven ST of 2.9% at three years of follow-up in a general population and the incidence of late ST did not diminish, but continued at a steady rate of 0.6% per year during the first three years. Moreover, in a systematic review of 14 randomised clinical trials, in which 6,675 patients were randomised to DES vs. BMS, an elevated risk of late and VLST for DES was demonstrated despite the fact that the overall incidence of thrombosis was the same for both stent types; the rate of ST with DES seemed to be constant at 0.5% per year, while this rate with BMS decreased over time.19 In a meta-analysis with 5,030 patients, no differences existed in the overall incidence of ST between DES and BMS neither in the incidence of late ST according to the various definitions as proposed by the ARC.16 Pooled analysis of four randomised trials comparing sirolimus eluting stents and of five randomised trials comparing paclitaxel-eluting stents with BMS, revealed no significant differences in the cumulative rates of protocol defined ST between DES and BMS, but significantly more VLST for both type of DES.21 Although ST results in death or MI in most affected patients, the overall short- and long- term rates of death and MI have been found to be at least similar with DES and BMS.15,22 Moreover, in a pooled analysis from five randomised trials with a total of 827 diabetic patients treated with paclitaxel-eluting stents or BMS there were no significant differences between the two types of stents in the rates of death, MI, or ST, at 4-year follow-up, but treatment with paclitaxel-eluting stent was associated with a significant and durable reduction in TLR.23
We also confirmed previous studies reporting the relationship between ejection fraction and ST; ejection fraction < 40% was the only predictor for VLST and overall ST in our study. Iakovou et al24 reported that low ejection fraction was one of the independent predictors of ST (p<0.001 for each 10% decrease). In another study, among the baseline characteristics, ejection fraction <30% was predictor of ST.25 In a very recent study with 1,648 diabetic patients treated with either DES or bare metal stent, the two year angiographic ST occurred in 1.5% DES patients and 0.7% of BMS patients (p=0.18); one of the most powerful predictors of 2-year MACE was ejection fraction <35%.26
ST is considered as a multifactorial occurrence that has been attributed to a range of angiographic, technical and clinical factors,27 such as bifurcation lesions, acute coronary syndromes, renal failure, DM, total stent length, multivessel disease, discontinuation of APLT and low ejection fraction.24,28-30 Diabetic patients have a number of haematological disturbances that predispose them for an enhanced risk of thrombotic events due to increased platelet aggregation.31 DES implantation in this population remains effective, but clinical efficacy appears to be reduced by the occurrence of ST between one and two years.32
Antiplatelet treatment and DES thrombosis
Clinical data revealed certain patient-related risk factors for VLST such as diabetes, low ejection fraction, or discontinuation of APLT.24 Withdrawal of dual APLT has been reported as a cause of ST after DES implantation.18,24,33 Most of patients (72%) in our study continued dual APLT beyond the first 12 months after the index procedure. The practice of prolonged dual APLT in a high percentage of patients combined with the fact that there were no definite or probable ST by ARC definitions may suggest that in the presence of continued dual APLT, true stent thrombosis with DES is very rare and that the deaths seen in long-term follow-up may be the result of other causes unrelated to ST. Despite the fact that dual APLT is recommended to prevent ST, not enough information is available on the utility of dual APLT over a long period. In a study with 3,021 patients treated with DES, conducted to establish the role of dual APLT in preventing ST, discontinuation of dual APLT was the most powerful predictor of ST during the first six months after stent implantation, but discontinuation of thienopyridine therapy after six months from DES implantation was not a predictor of ST.34 In another large cohort of patients receiving DES or BMS, the long-term risk for death and MACE was significantly increased among patients in the DES group who had discontinued clopidogrel therapy at six or 12 months, while extended duration of clopidogrel therapy following DES implantation was associated with a lower incidence of death
or MI.25 In a series of angiographically documented cases of VLST, no cases occurred in patients who were receiving dual APLT.35
Study limitations
This is a single centre non-randomised study with the decision about stent type determined by the availability of the particular type of stent as well as physicians discretion. Paclitaxel eluting stents and zotarolimus eluting stents were used one and two years later, respectively, than were sirolimus eluting stents.
The use of the definitions of possible ST proposed by the ARC may overestimate the actual rate of VLST. Indeed, all of the VLST events were possible events – that is, SCD. Some deaths may have come from a wide variety of other causes such as a fatal arrhythmias or coronary artery disease progression, not rare in diabetic patients, and not from ST due to DES implantation. Moreover, cardiac mortality is increased in patients with depressed left ventricular function, regardless of DES implantation. Therefore, given the limitation of definitions of possible stent thrombosis, the actual rate of VLST is even lower than that referred to in our results.
A comparison with diabetic patients after BMS implantation was beyond the scope of this study. The exclusion of patients without completion of 12 months of follow-up may have biased our results for the incidence of early and late ST.
Conclusions
DES implantation is an effective and safe treatment of coronary artery disease in DM patients. The cumulative incidence of overall ST and VLST during long-term clinical follow-up is low. An independent predictor for this complication is depressed left ventricular systolic function. Prolonged dual APLT treatment seems to minimise the risk of late and very late ST in this high-risk group of patients undergoing PCI with DES.

