Abstract
Animal models facilitate our understanding of human disease by providing a controlled environment permitting testing of mechanisms of disease, diagnostic technologies and therapeutic interventions. The ideal animal model should display coronary lesions resembling those seen in human atherosclerosis. No suitable large animal model of high-risk (vulnerable) plaque exists. Lack of such a model has hampered studies designed to validate imaging technologies and to scrutinise the effects of therapeutic interventions in atherosclerotic arteries. Several porcine models of advanced human-like coronary atherosclerosis exist. In this review some of the most promising porcine models are discussed, focusing on their applicability in the development and validation of coronary imaging technologies and interventional devices. In the evolving era of technological development, the availability and use of such animal models of advanced human-like coronary atherosclerosis and vulnerable plaque will become critically important in the preclinical testing of emerging technologies in interventional cardiology.
Introduction
Animal models facilitate the understanding of human disease when important aspects of the disease process develop in a predictable time period within a controlled environment. Although no animal model can fully replicate complex human pathological conditions, animal models are key for the evaluation of mechanisms of disease and testing of diagnostic technologies and therapeutic interventions1-3. Specifically, animal models of atherosclerosis and high-risk (vulnerable) plaque are pivotal in the development and validation of novel imaging technologies. Such validation has been hampered by the lack of a suitable large animal model of human-like coronary atherosclerosis1,4. Historically, non-atherosclerotic porcine coronary injury models have been used extensively in the validation of treatments against restenosis, and device development. However, current diagnostic and therapeutic devices should be tested in more clinically relevant animal models. Pigs harbouring metabolic conditions such as hypercholesterolaemia or diabetes develop atherosclerotic plaques in anatomical locations that are similar to the human condition. Depending on swine breed, cholesterol levels, exposure time or the presence of vascular injury, advanced and heterogeneous coronary lesions displaying humanlike features may develop in a relatively short period of time. This paper reviews the current status of several porcine models of coronary atherosclerosis and vulnerable plaque and discusses the applicability of these models for testing and validation of emerging coronary imaging technologies.
Swine models in biomedical research
Genetically, the pig is relatively close to humans5. This also applies to the anatomy and physiology of the cardiovascular system and the arterial response to hypercholesterolaemia. The domestic crossbred farm pig, sus scrofa domestica, is the most commonly used swine in cardiovascular research today2,3,6. However, in situations in which significant growth would be problematic, miniature swine (Yucatan, Hanford, Gottingen and Sinclair Hormel breeds) are often used in chronic studies. Typically, swine achieve sexual maturity by six to eight months at which time their weight usually varies between 40 and 100 kg. The heart of the pig is anatomically similar to the human heart with the exception of having a left azygous vein draining the intercostal system into the coronary sinus7 and a right coronary artery ostium situated substantially more cranially. The heart of a 40-50 kg miniature pig is approximately the same size as an adult human heart8. The coronary artery system is similar to 90% of the human population in anatomy and function, is more prone to vasospasm during manipulation, and possesses no pre-existing collaterals between the coronary arteries and their branches9.
Developmental aspects of swine models for atherosclerosis
Several important biological and anatomical factors must be considered before selecting the appropriate animal model in the setting of technology validation. For testing of emergent imaging techniques, the developed atherosclerotic lesions should contain the basic histological components of interest, such as a large necrotic core, a thin fibrous cap, inflammatory cells, and/or angiogenesis10,11. Alternatively, for stent validation studies, more advanced and fibrotic coronary lesions may be more desirable. Focal atherosclerotic lesions located in accessible regions are desirable. The reproducibility of the model should avoid the screening of multiple animals in an attempt to isolate a cohort sharing desired anatomical features. Normal swine on normal feed have low plasma LDL and relatively high HDL cholesterol levels and, consequently, do not develop mature atherosclerotic lesions. However, when fed a cholesterol-rich diet swine will develop hypercholesterolaemia (±300 mg/dl) and atherosclerotic lesions similar to those seen in humans. The evolution and histological types of lesion developed depend on swine breed, cholesterol level, exposure time, and coexisting proatherogenic conditions. Complex atheromatous lesions may eventually develop, but usually require long development times including months of severe hypercholesterolaemia. Although atherosclerotic plaque rupture and luminal thrombosis occur they are uncommon12-14. As in humans, diet-induced atherosclerosis in swine is multifactorial and results in the development of multifocal lesions of variable composition in unpredictable locations. A significant challenge facing animal model development is to shorten the typical 6-9 month period required to develop complex atherosclerotic lesions. Through the appropriate selection of swine breed, animals highly sensitive to dietary manipulation have been shown to develop complex lesions in a slightly accelerated time period (Table 1).
Porcine inherited hyper-LDL-cholesterolaemia model
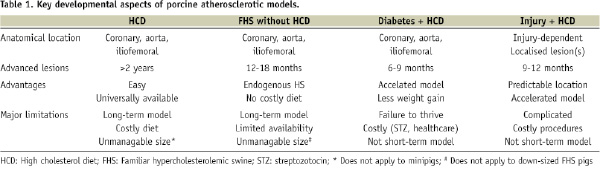
Rapacz et al originally described a strain of large domestic swine which develops elevated LDL cholesterol levels and spontaneous atherosclerosis while on a low-fat diet due to a mutation in genes coding for apolipoproteins and/or the LDL receptor15,16. As a result these pigs develop human-like atherosclerotic lesions on normal feed. By 12 months of age, early atherosclerotic lesions composed of macrophage-derived foam cells and smooth muscle cells are seen in the most susceptible arteries (coronary and iliofemoral arteries and aorta). However, a rapid progression of disease between 12 and 18 months is observed and well-developed atheromas are found in 60% of the pigs by 18 months (Figure 1).
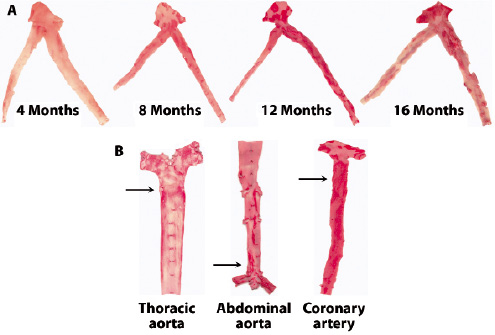
Figure 1. Natural history of atherosclerosis in the familiar hypercholesterolaemic swine (inherited hyper-LDL-cholesterolaemia). Lipid-rich lesions are stained by Oil Red O. A: Time-dependent development of lesions in the left main, left anterior descending (LAD) coronary artery and circumflex branch. At 16 months of age, advanced lesions are predominantly seen proximal in the coronary arteries. B: Disease distribution: At 18 months, lesion development is predominantly in the proximal thoracic aorta, distal abdominal aorta and its trifurcation, and proximal coronary arteries (arrows). (Figure courtesy of Chris Krueger and Jess Reed at the Department of Animal Sciences, University of Wisconsin, Madison).
Peripheral arterial lesions are more frequently found in the thoracic aorta and aorto-iliac bifurcation and tend to be more fibrous than the coronary lesions. By 24 months, complicated stenotic lesions containing fibrous caps, necrotic cores, cholesterol clefts, granular calcium deposits, and neovascularisation deep within the lesion are almost universally present in the major coronary arteries (Figure 2).
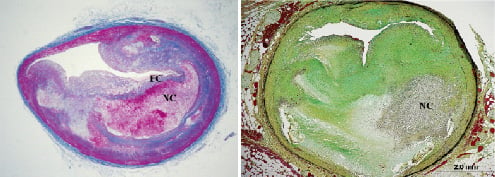
Figure 2. Advanced human-like coronary lesions in familial hypercholesterolaemic swine (inherited hyper-LDL-cholesterolaemia). Left: Atheromatous lesion containing a large necrotic core (NC) covered by a fibrous cap (FC) that is very thin near the shoulder region (vulnerable plaque). Right: Complex stenotic lesion containing collagen, lipid, calcification, and inflammation, predominantly macrophage foam cells. Collagen is blue in left panel (Masson stain), green in right panel (Movat stain).
By 36 months, plaque neovascularisation and haemorrhage are common, and plaque rupture may occur. Luminal thrombosis appears to be rare and acute occlusive thrombosis has not been documented, however, sudden cardiac death is common beyond three years of follow-up (personal communication JFG).
Atherosclerotic lesions in this model are characterised by marked plaque neovascularisation, a finding that can be exploited to test imaging modalities that detect this feature. Necrotic core formation and eccentricity of lesions in this model are also very similar to human disease, however, media destruction is comparatively less pronounced. Although this swine model develops humanlike atherosclerosis while maintained on a regular diet, a major limitation is the duration necessary for advanced coronary lesions to develop (>1 year) and the subsequent difficulties encountered in handling the mature animals, whose weights can exceed 200 kg. A cholesterol enriched diet might accelerate the process and thus mitigate these limitations but, unfortunately, no such information is available. Ongoing efforts are being undertaken both in the US and Europe to downsize the spontaneously hypercholesterolaemic pig to a more manageable size which will increase its usefulness substantially.
Porcine diabetes/hypercholesterolaemia model
Gerrity et al demonstrated that the combination of induced diabetes and hypercholesterolaemia (DM/HC) in Yorkshire pigs led to advanced atherosclerotic lesions in the coronary, femoral arteries and aorta in a relatively short period of time (20 weeks)17. Diabetes was induced with an intravenous infusion of streptozotocin resulting in a >80% reduction in pancreatic beta cells. Following administration of a high cholesterol diet, accelerated atherosclerosis was observed. Diabetic only animals did not develop atherosclerosis17 but the combination of diabetes and hypercholesterolaemia led to accelerated development of more advanced lesions. Early atherosclerotic lesions developing within three months are generally intimal xanthomas18. By six months, 96% of arteries harbour an atherosclerotic lesion with progression to 100% at nine months post induction. At 9-months many arteries demonstrated complex human-like lesions with the presence of fibroatheromas (17%) and calcific lesions (11%, Figure 3)17,18.
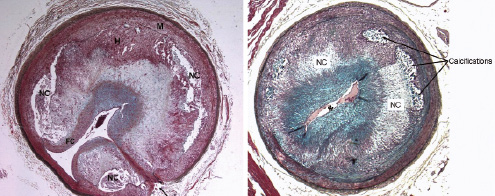
Figure 3. Advanced lesions in diabetic+hypercholesterolaemic (DM/HC) swine. Left, 6 months after DM/HC induction: Complex stenotic plaque in an iliac artery containing well-developed necrotic cores (NC) covered by a fibrous cap (FC). Intraplaque haemorrhage (H) is also seen. Arrow indicates a side-branch and tunica media (M). Right, 9 months after DM/HC induction: Coronary fibroatheroma with large necrotic cores (NC) and heavy calcifications near the internal elastic lamina which is destroyed from 8 to 10 o’clock and at 12 o’clock. The lumen (*) is severely narrowed. Both Movat stain.
As in humans, the most severe lesions were generally located in the proximal segment of the coronary arteries often at the side-branch points. Chatzizisis et al demonstrated that areas of low endothelial shear stress in coronary arteries were associated with subsequent development of lesions. These lesions had increased lipid accumulation, inflammation and expansive remodelling; all characteristics of high-risk (vulnerable) atherosclerotic lesions19. Focal calcifications were noted at 6-9 months as well as thin-cap fibroatheromas in some arteries. More advanced lesions possessed acellular necrotic cores covered by fibrous caps with medial thinning, haemorrhage and calcification (11% thin-cap fibroatheromas)18. Treatment with darapladib, a selective inhibitor of Lp-PLA2 , an important mediator of vascular inflammation has been shown to reduce development of atherosclerosis but more importantly necrotic core development in this model20.
As with humans the development of atherosclerosis in DM/HC is variable with regard to location, severity and extent. Coronary arteries develop lesions more often and more severe than within the peripheral arterial territory17,18. Carotid arteries showed more resistance to lesion development under DM/HC conditions. The proximal iliac artery is also predisposed to lesion development (Figure 3). Studies of gene expression have shown that inflammatory genes were more markedly upregulated in coronary arteries than thoracic aorta and carotid arteries from the same animal18. This may reflect the presence of pulsatile flow and significant number of branching arteries in the coronary circulation. A distinct advantage to the DM/HC model is that diabetic pigs gain weight at a slower rate than hyperlipidaemic or non-diseased pigs. Limitations of the model are the variability in size and location of the developed lesions and the expense in maintaining the model. In addition, DM/HC animals can develop hypoglycaemic coma if not closely monitored and are prone, as are human diabetics, to infections and gastroparesis. A DM/HC model has also been described in Yucatan minipigs, but plaque size was only reported for a few pigs21.
Minipig models of coronary atherosclerosis
Atherosclerosis is a chronic disease, and it takes relative long time to develop advanced lesion in pigs similar to those responsible for clinical disease in humans. The rapid growth of the domestic swine makes it less suitable for long-term studies compared to minipigs, not only because of the high maintenance costs but also because of the continued growth (non-steady coronary dimensions) leading to a large and heavy pig that is difficult to image (loss of resolution) and handle. Some colonies of Yucatan minipigs have proved to be susceptible to both diet-induced hypercholesterolaemia and atherosclerosis, and they have been used for decades in atherosclerosis and restenosis research13,22-25. Complicated human-like atherosclerotic lesions displaying necrosis, cholesterol crystals, and calcification may develop relatively rapidly on a cholesterol-rich atherogenic diet13. Furthermore, streptozotocin-induced diabetes has been described, and preliminary data indicate that it accelerates diet-induced coronary atherosclerosis and the development of vulnerable plaques in Yucatan minipigs as it does in Yorkshire swine21. Other minipigs, such as the Göttingen, Hanford and Sinclair Hormel breeds, have also been used in atherosclerosis research but far less information concerning their use is available compared to the Yucatan mini/micro swine. Despite great enthusiasm a few years ago, human-like atherosclerosis has not yet been documented in the obese, insulin-resistant, mildly hypertensive, hypercholesterolaemic Ossabaw pig26.
Porcine vascular injury/hypercholesterolaemia models
In order to shorten the time required to develop lesions of interest and limit the high maintenance costs, multiple attempts have been made to accelerate the atherosclerotic process by combining diet-induced hypercholesterolaemia with a localised vascular injury such as external radiation27, wire-induced endothelial denudation28, barotrauma24,25,29, or haemodynamic manipulations30. In 2001, Keelan and Schwartz introduced the concept of “accelerating” the atherosclerotic process locally by combining balloon injury (Infiltrator catheter, Boston Scientific, Minneapolis, MN, USA) and triggering inflammation by directly injecting a large lipid pool into the vessel wall of hypercholesterolaemic pigs31. One variation of this approach involves the delivery of a large concentration of lipids into the adventitial layer of normal porcine coronary arteries using a specialized micro-needle catheter32. With this technique excessive vascular trauma secondary to overstretching was avoided by direct injection. Feasibility studies demonstrated safety and efficacy of this technique32. Short term studies (eight weeks) demonstrated that intramural delivery of lipids initiate the process of atherosclerotic disease. By seven weeks, IVUS analysis of the injected segment showed that the lipid injection induces the formation of early eccentric fibrotic lesions located in positively remodelled segments. By histology, the lesions are eccentric, non-obstructive and contain large amounts of lipid co-localising with foam cells. After 12 weeks of delivery, the lesions continue to develop and are more significant in size and contain increased amounts of lipid and macrophages33. At 12 months, these atherosclerotic lesions show a necrotic core covered by a dense fibrous cap containing foam cells and abundant intraplaque neovessels. The locally induced lesions in this model are characterised by the rupture of the elastic components of the vessel and abundant adventitial plaque neovascularisation (Figure 4).
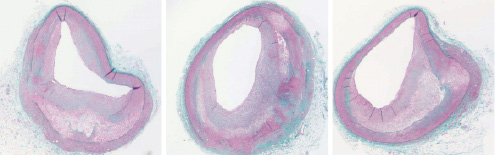
Figure 4. Advanced coronary lesions at predictable location (proximal coronary arteries) after local injury (lipid injection) in hypercholesterolaemic swine. As illustrated, coronary fibroatheromas with necrotic cores covered by fibrous caps are common in this model. Inflammatory cells, including macrophage foam cells, are usually seen in the fibrous cap at high magnification (not shown). Collagen is green-blue in this polychrome staining.
Interestingly, due to the high cholesterol diet, staining of the surface of the vessels usually displays early atherosclerosis in the thoracic aorta, distal abdominal aorta and proximal coronary arteries. Compared to balloon injury or saline injection, the injected vascular segments demonstrate increased expression of chemokine mRNA and chemokine receptors important in macrophage recruitment34. An important feature of this model is that the anatomical distribution of the lesions is predictable as it follows the pattern of injury induced. Therefore, it provides an opportunity for selecting the anatomical targets and optimising the sample size. The major limitation of this model is the complexity of lesion induction requiring mechanical intervention and diet supplementation. Although the development time is reduced, it still takes up to one year until advanced coronary lesions are evident. Additionally, the barotrauma associated with the delivery device may produce a disproportionate component of neointimal hyperplasia35. In order to decrease the development time even further, several groups are attempting to accelerate the atherosclerotic process by inducing mechanical injury in the porcine inherited hyper-LDL-cholesterolaemic model.
Research applications of swine models of atherosclerosis
In this review we have tried to highlight the fact that pig models may develop humanlike coronary atherosclerosis if exposed to atherogenic stimuli for at least several months. Plaque size and composition depend on the method used to induce the lesion. High-risk plaque features assumed to confer “vulnerability” in humans are also seen in advanced porcine lesions, including a large necrotic core, a thin fibrous cap, inflammation, angiogenesis and plaque haemorrhage. The ultimate proof of “vulnerability” is, however, usually lacking, because plaque rupture is uncommon and luminal thrombosis is rare. Despite this deficiency, porcine models of coronary atherosclerosis and morphologically vulnerable plaques can provide meaningful information in the development, validation, and improvement of novel diagnostic and therapeutic technologies for use in interventional cardiology.
Novel imaging technologies to detect atherosclerosis, specifically high-risk (vulnerable) coronary artery lesions, are being evaluated in both humans and animal models. For validating non-invasive diagnostic technology, swine models provide suitable size, vascular anatomy and physiology similar to human disease. Indeed, the majority of the initial validation work on CT angiography and contrast media injection and MRA compatible contrast agents36,37 has been developed in the normal porcine model38-41. In this field, porcine models have been previously used in studies requiring the presence of arterial obstructions42,43 or the development of acute myocardial infarction44. Particularly, porcine models have been very useful in the evaluation of PET related technologies45-48 and molecular tracers49-51. In the field of invasive cardiovascular imaging, animal models are relevant as it is important to document and characterise morphology, location and presence of specific structural plaque components such as calcium. Most of the initial IVUS52 and OCT technology development53,54 involved the use of the normal porcine coronary model. However, as the endovascular imaging technology field evolves, the use of the normal porcine coronary model will be no longer viable. Specifically, the characterisation of specific biological components within the vessel wall including remodeling55, plaque strain56 or tissue components57 have required the use of injury based animal models. These models may be critical during the validation process of imaging technologies designed to detect specific biological components (i.e., IVUS based tissue characterisation and vasa vasorum imaging) encountered in human atherosclerotic lesions (Figure 5).
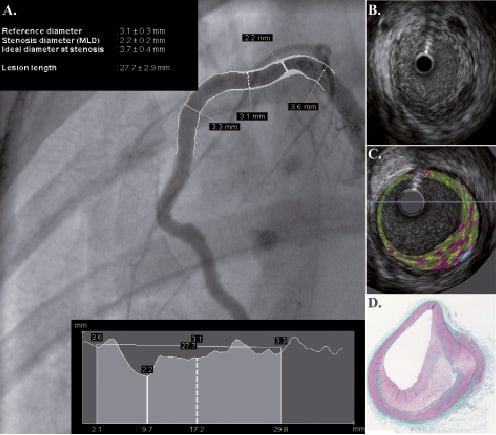
Figure 5. IVUS-based tissue characterisation performed in swine. A non-obstructive lesion is visualised in the proximal segment of the RCA located in a previously injured segment. After injury, swine were maintained on a high cholesterol diet for 9 months. Plaque evaluation by QCA (A), IVUS (B), IVUS-based tissue characterisation (C) and histology (D).
Finally, in the area of cardiovascular interventions, healthy swine have been traditionally used as the animal model to test endovascular technology. In the evolving era of drug eluting stents, for example, a large animal with pre-existing metabolic and/or arterial disease may be needed to validate potential therapeutic strategies and their long-term consequences, such as demonstrating the effects of therapies directed toward limiting vascular inflammation and necrosis, preserving the endothelium, and promoting healing23,58,59.
Conclusions
Clinical implementation of innovative diagnostic and therapeutic technologies will ultimately depend on the successful development of large animal models of humanlike coronary atherosclerosis that permit preclinical validation of the technology in a controlled environment. General limitations in porcine model development are well recognised and include the need for significant infrastructure for animal maintenance, the long time period needed to induce and monitor the lesions, and development costs. In current models of “naturally” occurring atherosclerosis (genetic, diet-induced), significant coronary artery disease occurs late and is unpredictable in location. Hence, further model development is needed, including innovative ways to accelerate the atherosclerotic process in susceptible animals with or without pre-existing arterial disease. The current limitations notwithstanding, valuable porcine models of atherosclerosis are already available for development, validation, and improvement of imaging technologies. How reliably these porcine models mimic the chronic pathobiology and natural history of human atherosclerosis requires further exploration. Regardless, the availability of animal models of human-like atherosclerosis will become perhaps the most critical element of the preclinical validation of emerging diagnostic and therapeutic technologies developed for use in patients with coronary artery disease.
Acknowledgements
The authors are thankful and extend gratitude for the research collaboration and support of Chris Krueger and Jess Reed at the Department of Animal Sciences, University of Wisconsin, Madison. The authors would like to thank David Wallace-Bradley for his assistance in the preparation and editing of this manuscript.

