Abstract
Aims: To assess the safety and efficacy of the XTENT® customisable drug-eluting stent system in the treatment of patients with single long or multiple coronary lesions referred for PCI.
Methods and results: The CUSTOM-II trial enrolled 100 patients with de novo lesions in native coronary arteries presenting with either single long lesions (n=69) of > 20 mm length or up to two lesions with a total cumulative anticipated stent length of 60 mm of stent (n=31). Patients were assessed angiographically at six months, and clinically at one year.
Of the 100 patients enrolled, nine patients experienced a MACE, including five patients whose MACE occurred during index hospitalisation (two non-Q-MI, two Q-MI and one probable stent thrombosis-related death), and four target lesion revascularisations (TLR) at six months. No MACE or stent thrombosis was reported between six and 12 months follow-up. In-segment late loss at 6-months was 0.22±0.28 mm, and in-stent late loss had a range of 0.31±0.31 mm.
Conclusions: The XTENT® customisable stent is clinically safe and efficacious as judged by angiographic and clinical variables through 12 months follow-up. Further follow-up and larger randomised comparative studies are needed for its clinical positioning.
Introduction
Drug-eluting stents (DES) are the current standard of care for the percutaneous treatment of coronary artery disease. Recently, there have been concerns regarding the occurrence of late stent thrombosis in the first generation of DES. In these stents, the drug carrier consists of a durable polymer that remains intact even after the drug is completely eluted and this in turn could elicit a chronic inflammatory response, delayed healing and (very-) late stent thrombosis.1-6
Fully biodegradable polymers on the next generation of DES should degrade following drug delivery and leave behind only a bare metal stent, thus potentially reducing the rate of stent thrombosis. The combination of the polylactic acid (PLA) carrier and the Biolimus A9 drug has shown promising results in both the prevention of clinically-driven target lesion revascularisation (TLR) and the reduction of stent thrombosis.7,8
Although first generation DES have brought significant advances to the field of interventional cardiology, current stent designs and methods of delivery can limit their effectiveness especially during the treatment of long and multiple coronary lesions. This procedure is complex and often results in suboptimal angiographic results and increased radiation exposure to both the physician and to the patient. Furthermore, multiple delivery systems and stents that are being used result in increased costs for healthcare providers.
The XTENT® Custom-NX Catheter System (Xtent Inc, USA) allows for in situ customisable implantation of balloon-expandable stents of up to 60 mm. The system enables the physician to adjust the stent length to the appropriate length of lesion to be treated within the coronary artery. This in situ customisation occurs in the same procedural setting as the stent implantation and does not require the physician to predetermine the stent length. Additionally, due to the unique design of the XTENT® Custom-NX System, the risk of inappropriate lesion coverage and stent overlap is eliminated.
Methods
Patients
Patients were enrolled at 10 centres across four countries (see appendix for all participating study sites). The study protocol was approved by the institutional ethics committees, all patients provided written informed consent, and the study complied with the European-recognised Standard ISO-14155 and the Declaration of Helsinki. Those eligible for balloon angioplasty with symptomatic ischaemic heart disease, characterised by de novo coronary artery lesions in reference vessel diameters from 2.5 mm-3.0 mm were included in this study, provided all inclusion criteria were met. The main criteria included de novo coronary lesions (no internal mammary or saphenous vein grafts) in two main categories: either single, long lesions > 20 mm in length or multiple lesions with a cumulative lesion length needing a maximum of 60 mm of stent. Of the multiple lesion cohort, up to two lesions could be treated. Table 1 summarises the main inclusion and exclusion criteria.

Device description
The XTENT® Custom-NX 60 DES system is comprised of multiple stent segments and an integrated balloon-inflatable delivery system, allowing for the treatment of reference vessel diameters of 2.5 mm to 3.0 mm. The system comes in three different sizes (2.5-3.0-3.5 mm) but for this study only the 3.0 mm device was available and used. The stent is balloon expandable and protected by an outer sheath. Each stent segment is 6 mm in length with a total of 10 segments. The segments have an interdigitated design that permits excellent scaffolding between segments upon balloon inflation. Each single stent segment is composed of a cobalt chromium mesh frame, a primer layer of inert material (parylene C) and the drug-eluting coating formulation. By manipulating the components of the delivery system, the operator can control the number of stent segments to be deployed in situ, allowing for a customised stent length to match the lesion length. The semi-compliant delivery balloon of the device allows for achievement of up to 0.5 mm difference in stent diameter at the same coronary segment, therefore the distal tapering in the treatment of a long lesion has not been a clinical issue. The delivery system of the device is illustrated in Figure 1.
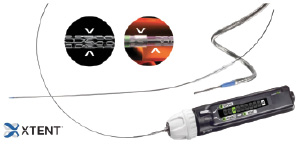
Figure 1. XTENT® Custom-NX platform delivery system.
The drug-eluting coating formulation used in the Custom NX DES System combines the Biolimus A9 drug (93.6 mg/segment) together with polylactic acid (PLA) coating. Biolimus is modified at position 40 of the rapamycin ring to enhance its lipophilicity and elution rate. The rapamycin ring structure binds to an intracellular receptor, the FK506 binding protein (FKBP-12), forming the macrolide/FKBP-12 complex that subsequently binds to mTOR (mammalian target of rapamycin). By this means, Biolimus interrupts intimal cell migration and proliferation by arresting the cell cycle in the late G1 phase. In addition, the presence of asymmetric PLA polymer coating on the study stent presents an advantage over durable polymers, eliminating a possible chronic source for inflammatory reactions and thrombosis after the drug has eluted, as was mentioned above. The PLA carrier used is completely absorbed by approximately 12 months, leaving only the bare metal stent and thereby reducing the risk of any late adverse events. PLA has a controlled biodegradable profile, degrading first into lactic acid, a natural metabolite, and finally into carbon dioxide and water.9,10
Device implantation
Subjects meeting the eligibility criteria underwent physical examination and ECG testing. Baseline angiography of the target vessel was performed and the estimated length of the target lesion was determined as well as other angiographic characteristics of the target lesion, including morphology and classification. The guiding catheter used during the procedure had a minimum internal diameter of > 6 Fr. Prior to treating the target lesion, standard percutaneous transluminal balloon angioplasty was performed to predilate the stenotic area at the discretion of the operator. Following the placement of a standard guidewire, the XTENT® Custom-NX system was delivered to the site of the target lesion. Opaque markers on the balloon inflation lumen and the protective sheath allowed for accurate positioning of the catheter. Features of the device’s handle enabled the operator to control the number of stent segments to be deployed and also to separate the exposed stent segments from those remaining protected under the sheath of the catheter. If desired, for better stent apposition, the operator modified the length, repositioned and re-inflated the balloon in situ. For those in the multiple lesion cohort, the XTENT® Custom-NX DES system was reset and used to treat the additional lesion with the remaining stent segments. An example of a patient with two long coronary lesions in RCA that was treated with the XTENT® Custom-NX DES system, is shown in Figures 2-6.
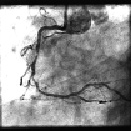
Figure 2. Baseline angiography of a patient with two coronary lesions in distal and in proximal RCA.
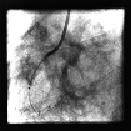
Figure 3. XTENT® Custom-NX delivery system is used in order to treat the distal lesion in RCA.
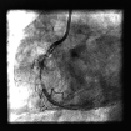
Figure 4. After the stent placement in distal RCA , the XTENT® Custom-NX system is reset and used to treat the long proximal lesion with the remaining stent segments.
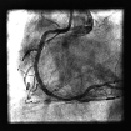
Figure 5. Final angiographic result.
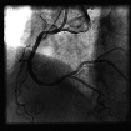
Figure 6. Follow-up angiographic result of the RCA six months later.
Prior to the index procedure, at least 75 mg of aspirin QD for at least seven days prior to the procedure and 300 mg clopidogrel or 500 mg ticlopidine per os were given. During the procedure, heparin was given by IV per hospital protocol. Intracoronary nitroglycerin 50-200 µg bolus was given prior to baseline angiography and post-intervention. Two or more orthogonal views were taken to show non-foreshortened views of the target lesion. Quantitative coronary angiographic and IVUS endpoints were assessed after PCI and at six months included binary restenosis, late loss, loss index, late absolute minimal luminal diameter (MLD) and minimal lumen area (MLA). Post-procedure, 75 mg clopidogrel or 250 mg ticlopidine were recommended daily for a minimum of 90 days post-stent implantation.
Study endpoints - primary endpoint
The primary endpoint for this study was the rate of major adverse cardiac event (MACE) at six months. MACE was defined as cardiac-related death, any myocardial infarction, and clinically-driven target lesion revascularisation. Q-wave MI was defined as the development of new, pathological Q waves in two or more contiguous ECG leads (as assessed by the ECG core laboratory) with post-procedure CK or CK-MB levels elevated above normal. Non-Q-Wave MI was defined as elevation of post-procedure CK levels > 2.0 times normal with elevated CK-MB in the absence of new pathological Q waves (WHO definition).
Secondary endpoints
Secondary endpoints for this study included angiographic endpoints, device performance endpoints and other safety endpoints. Performance endpoints included procedural success, defined as the achievement of a final diameter stenosis of < 15% (visual estimate) without an occurrence of MACE during the hospital stay. Successful device performance was defined as the successful delivery and deployment of a customised stent to the target lesion covering the total lesion length and achieving a final diameter stenosis of <15% by visual estimate.
Secondary safety endpoints include MACE rate at 30 days and 12 months, the incidence of bleeding and/or vascular complications, the incidence of (sub)acute stent thrombosis (SAT) at 30 days and stent thrombosis at 12 months. Stent thrombosis definition was revised during the course of the trial to adopt the newly recommended ARC definitions11. All events were therefore reviewed and adjudicated by an independent clinical events committee per those definitions. All patients in both trials underwent clinical follow-up at 30 days, six and 12 months and subsequently will undergo clinical follow-up annually for five years. All data points were monitored by an independent monitoring CRO and all events were adjudicated by an independent Clinical Event Committee.
QCA & IVUS
Quantitative coronary angiography analysis (QCA) and intravascular ultrasound analysis (IVUS) were performed by two independent core laboratories (see appendix). In three patients an OCT study was performed at follow-up, showing excellent results with minimal neointimal formation and no signs of late malapposition (Figures 7 and 8).
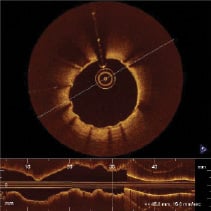
Figure 7. OCT image at six month follow-up in the mid-part of a long stented lesion.
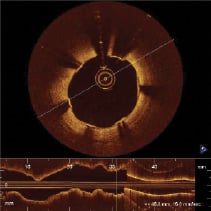
Figure 8. OCT image in the proximal part of a long stented lesion at six month follow-up.
Statistics
Categorical variables were summarised using counts and percentages. Continuous variables were summarised using mean, standard deviation, minimum value, maximum value, and median. Rate of MACE at twelve months is presented with a 95% confidence interval and in hierarchical manner. All safety data are summarised as part of intent to treat analysis.
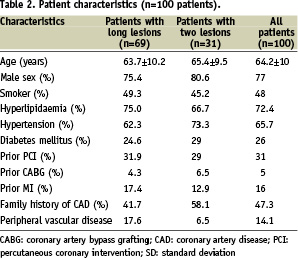
Results
The CUSTOM II trial enrolled 100 patients, using the XTENT® Custom-NX DES system and enrollment was completed in October 2006 at 10 cardiology centres in Europe. Two cohort groups represented single long lesions treated (n=69) and multiple lesions (n=31). The mean age of the population was 64.2 years. 77% were men and 26% were diabetics, 72.4% had a history of hyperlipidaemia, 65.7% hypertension, 36% were previously revascularised (PCI or CABG) and 16% presented with a history of myocardial infarction. Lesion characteristics demonstrated more complex morphology as 65.1% were B2/C grade lesions according to the ACC/AHA classification.
Patient and lesion characteristics in the CUSTOM-II trial are summarised in Tables 2 and 3.
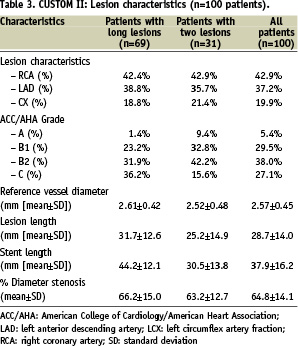
Mean lesion length was 28.7 mm with a mean stent length implanted of 37.9 mm, representing the “real world” difficult lesion subset. The distribution of the stent length for the single long lesion cohort (n-69) is provided in Figure 9.
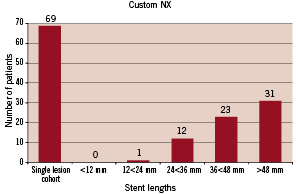
Figure 9. Distribution of the stent length used in the single-lesion cohort of patients.
For each length group represented one may deduct the number of used segments in the system in order to calculate the discarded segments. In the multiple lesions cohort, half of the patients were treated with one Custom-NX system. The rest of them were treated with the use of two devices. A second device was utilised when crossing from one coronary tree to another where rewiring was necessitated, because at the time of enrolment the device was not validated to be removed from the circulatory system of the patient and then reinserted. In several patients also a second device was utilised because of inadequate lesion length coverage with a single device.
Clinical follow-up at one year was completed in the entire cohort (100% of patients) and is reported in Table 4.
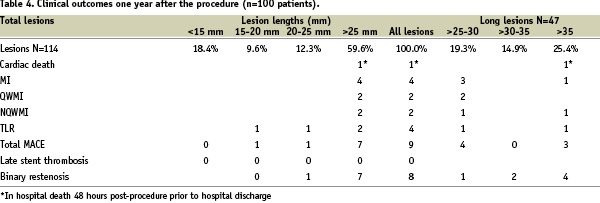
Ninety days of dual antiplatelet therapy was recommended per protocol to patients that were enrolled in the CUSTOM-II study. At one month follow-up ninety seven (98%) patients were receiving dual therapy, aspirin and clopidogrel. At six month follow-up, ninety-one (92%) patients were receiving dual antiplatelet therapy. At 12 month follow-up, 70% of all patients were taking clopidogrel. Procedural success was achieved in 98% of the cases and there were no device-related complications. There was one hospital death 48-hours post procedure, prior to hospital discharge, in a patient treated with a single long lesion, adjudicated as a probable stent thrombosis according to ARC definition. No other cases of stent thrombosis were recorded up to one year follow-up. There were four cases of non-fatal myocardial infarction during the index hospitalisation identified in patients with lesions greater than 25 mm. Four cases of clinically driven TLR were reported during the first six months follow-up, while there were no new events between six and 12 months after the procedure. Overall MACE rate at one year remained at 9%. At six months, clinical follow-up was completed for all patients (100%) and angiographic follow-up was performed in 90% of the patients. Follow-up QCA and IVUS results are reported in Table 5.
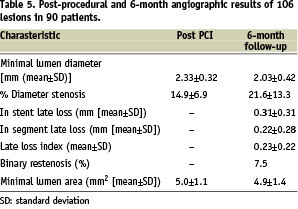
In-stent late loss was 0.31±0.31 mm and in-segment late loss was 0.22±0.28 mm. Patients will be followed annually for five years.
Discussion
The results of the CUSTOM II trial confirm that the XTENT® Custom NX stent is highly deliverable and appears safe and efficacious as judged by angiographic and clinical variables at 12 months follow-up both in single long, or multiple lesions. While most new stent platforms have been studied in predominantly simple lesions,12-14 CUSTOM II involved more “real-world” anatomical characteristics consisting of very long or multiple lesions.
Regarding in-stent late loss, the results of the biolimus-coated XTENT® Custom-NX compare favourably with those of sirolimus-eluting stents (late loss up to 0.20 mm)14-16 and paclitaxel-eluting stents (late loss 0.30-0.49 mm).7,17 This efficacy is accompanied by an excellent safety profile with an overall MACE rate of 9%. Remarkably, no late stent thrombosis was reported between six and 12-month follow-up, although only 70% of all patients were taking dual antiplatelet therapy at 12 month follow up. In addition to the Biolimus A9 drug, the presence of the biodegradable PLA polymer seems to be an advantage compared to the durable polymers used in the first-generation DES, potentially contributing to the low incidence of stent thrombosis.
In a recent randomised comparison of a DES platform using Biolimus-A9 with PLA coating versus Taxus Liberté®, the biolimus-eluting stent was found to be superior to the paclitaxel-eluting stent (PES) in terms of late loss at nine months (0.15±0.27 for BES vs. 0.32±0.33 for PES, p=0.006). Clinically-driven target lesion revascularisation was 0% in the biolimus group versus 2.9% in the PES group.7 The neointimal suppression induced by the Biolimus A9/PLA drug combination as demonstrated in CUSTOM-II, appears superior not only to paclitaxel but also to zotarolimus (ZES) and additionally resembles the suppression previously reported for sirolimus14-16 and everolimus-eluting stents (EES).13,18 In detail,
in the SPIRIT-II trial, six months in-stent late loss with EES for lesions < 28 mm was 0.11±0.27 mm.13 In the ENDEAVOR-II trial, in-stent late loss for the ZES group was 0.67±0.46 mm12. In a large trial that compared PES and SES in long lesions, six month in-stent late loss was 0.45±0.55 mm and 0.09±0.37 mm respectively (p<0.001 in favour to sirolimus-eluting stents).17 More data is needed regarding the treatment of long lesions with the newer zotarolimus and everolimus stents.
These favourable results of the CUSTOM-II trial confirm the clinical and angiographic benefits of the Biolimus A9/PLA formulation, while extending them in a patient population with very long and multiple coronary lesions. Although both lesion and stent lengths in the CUSTOM-II trial are much longer than in previously reported studies with a relatively smaller reference vessel diameter, there was no evidence of increased MACE or target vessel failure (TVF) with the use of long-stented segments. In an indirect comparison of the CUSTOM-II trial primary endpoint to the 9-month results from TAXUS-V and VI trials that also involved long coronary lesions, MACE rates after XTENT® DES implantation were much lower than those reported with Paclitaxel-eluting stents (e.g. 9% vs. 20.4% vs. 16.4% respectively), although mean lesion length treated was longer (28 vs. 25 vs. 20.9 mm respectively).19,20 Another positive finding was that these favourable outcomes in CUSTOM-II are noticed even in relatively small vessel diameters, again compared to TAXUS-V and VI (2.57 mm in CUSTOM-II vs. 2.65/2.82 mm respectively).19,20 In a recent study in which extensive DES coverage of coronary lesions was performed, (defined as >50 mm stent length), 6-month TLR was found to be 6.7% and MACE plus TLR was 13%. Further analysis showed that stent length was the only independent predictor of MACE plus TLR.21 In CUSTOM II, 1-year TLR in the XTENT® DES group was 4% and MACE was 9%; these rates are favourable compared to those mentioned in trials testing other DES platforms in long lesions.
Theoretically, this new stent platform offers some major advantages over contemporary practice. First, the current practice of stent length estimation from fluoroscopy is avoided by virtue of in situ customisation coupled with the ability to cover the entire lesion length (up to 60 mm), which is longer than all currently available DES stents. This reduces the risk for geographical and longitudinal miss, events which can occur with procedures involving complex and diffuse lesions, where the dilated coronary segments are often not adequately covered by multiple fixed-length stents. Next is the potential to avoid stent overlap, which has been proven to be an independent risk factor for MACE22. Medial necrosis, increased intimal growth, and in some cases adventitial inflammation, have been reported at the site of DES implantation, especially using paclitaxel-eluting stents.1,5,23 This increases the risk of stent thrombosis at sites of DES overlap, given that drug and/or polymer concentrations are significantly higher in the overlapped segments.2,22,25,26 Furthermore, the rate of peri-procedural myonecrosis in the TAXUS-V trial was found to be significantly higher in the subgroup of patients who received overlapping PES versus the control group (8.3% vs. 3.3% respectively – p=0.047), because of the compromised blood flow at the side branches which increases proportionally if multiple/overlapping stents are applied.20 Approximately 33-35% of patients enrolled in the SIRIUS and E-SIRIUS trials were treated with overlapping stents and an estimated 29% in TAXUS VI.13,19,27 The unique features of the XTENT® Custom-NX DES system minimise the risk of inappropriate or inadequate lesion coverage and eliminate the need to use multiple or overlapping stents.
Apart from avoiding stent-overlap, another major advantage of the XTENT® Custom-NX system is the avoidance of stent fracture due to the unique stent design. Stent fracture is likely to be affected by mechanical stress provoked by rigid structures. In a recent study in which 280 patients were treated solely with sirolimus-eluting stents, the incidence of stent fracture was 2.9% while in-stent binary restenosis and clinical TLR rates were very high in those patients that had stent fracture (37.5% and 50% respectively).28 In another study using SES, stent fracture was identified as a cause of in-stent restenosis in 10 out of 26 in-stent restenosis lesions (39%).29 Its predisposing factors may be vessel tortuosity and use of overlapping stents.29,30
Finally, the benefits of the XTENT® Custom-NX system will lead to reduced procedural times, resulting in diminished contrast dye administration, increased patient safety, and lowered costs, since multiple stent length devices and balloon catheters on stock could be avoided.
The favourable results of the one year follow-up of the CUSTOM II study, in a high-risk patient population with complex multivessel coronary artery disease, of whom 35% were diabetics, confirm the safety and all potential theoretical benefits of the XTENT® Custom-NX system. These long-term results also demonstrate the safety of in situ customisation to treat complex coronary lesions with a single catheter insertion and confirm the superior efficacy of the Biolimus-A9 drug with PLA biodegradable coating stent formulation.
Appendices
Study Organisation – Clinical Events Committee: Cardiovascular Research Foundation, 55 East 59th Street, 6th Floor New York, NY 1002; Steven Marx, MD, CEC Chairman; David Engel, MD, CEC Member; Kevin Dunsky, MD, CEC Member; Roxana Mehran, MD, Non-voting Member; Vijaya Kesanakurthy, MD, Clinical Safety Monitor; Raza Hijazi, MD, Clinical Safety Reviewer. Data coordinating centre: Angiographic core laboratory: Marco A. Costa, Cardiovascular Imaging Core Laboratories, University of Florida Health Science Center Jacksonville, 655 West 8th Street Jacksonville, Florida 32209, USA. Intravascular ultrasound core laboratory: Peter Fitzgerald, Stanford University, Cardiovascular Core Analysis Lab, 300 Pasteur Drive, Stanford, CA 94305, USA. Participating centres and investigators: University Medical Centre Utrecht: Pieter R Stella, George Pavlakis; Helios Klinikum Siegburg: Prof. Eberhard Grube, Ralf Mueller; Barmherzigen Bruder Krankenhaus: Karl Eugene Hauptmann; Sankt Katharinen Krankenhaus: Prof. Horst Sievert; Clinique Pasteur: Jean Fajadet, Bruno Farah; Centre Cardiologique du Nord: Bernard Chevalier, Philipe Guyon; Institut Hospitalier Jacques Cartier: Marie Claude Morice, Thierry Lefevre; OLV Hospital: Bernard De Bruyne; Charité-Campus Virchow Klinikum: Wolfgang Bocksch; Polyclinique les Fleurs: Philippe Commeau; Angiography Core Lab, Shands University of Florida: Marco Costa; IVUS Core Lab, Stanford University: Peter Fitzgerald; Clinical Event Committee, Cardiovascular Research Foundation: Vijaya Kesanakurthy.
Online data supplement
Video 1. Exposure of Xtent device in LAD
Video 2. Separation of stents
Video 3. Inflation
Video 4. After placement
Video 5. OCT run of long stented segment in LAD at 6 month follow-up

