Summary
Background: A 43-year-old woman with a 2-hour history of chest pain presented with an anterior infarction and was referred for primary percutaneous coronary intervention.
Investigations: Physical examination, electrocardiography, laboratory tests, coronary angiography, abdominal angiography, cardiac CT, histology, immunohistochemistry, intravascular ultrasound.
Diagnosis: ST-segment elevation anterior myocardial infarction.
Management: Coronary angiography, antithrombotic therapy, statin, angiotensin-converting enzyme inhibitor, thrombectomy, coronary artery bypass graft surgery.
How should I treat?
Presentation of the case
A 43-year-old woman with no previous cardiac history presented with a two hour history of central chest pain with radiation to the left arm unresponsive to sublingual nitroglycerin. A pre-hospital electrocardiogram (ECG) showed ST-segment elevation in the precordial anterior leads consist with an evolving anterior infarction and was referred to our hospital for primary percutaneous coronary intervention (PCI). On admission to the coronary care unit (CCU), the pain had almost completely resolved. A 12-lead ECG showed persistent, but to a lesser degree, ST-segment elevation in all precordial leads (V1 to V6), including the inferior leads (II, III and aVF).
The woman was of African origin and had a history of sickle cell disease. She had arterial hypertension, which was treated with a thiazide diuretic, and a positive family history of premature coronary artery disease. She reported no history of diabetes or dyslipidaemia, and was a non-smoker. Her blood pressure on admission was 135/ 65 mm Hg, and on physical examination there were no signs of heart failure.
Aspirin (300 mg), unfractionated heparin (5000 IU), and nitroglycerine as a continuous intravenous infusion had been administered in the ambulance. Clopidogrel bisulfate (600 mg bolus) was added in the CCU. Coronary angiography showed a significant stenosis with fresh thrombotic material in the left main coronary artery (LMCA) that extended towards the ostium of the left anterior descending artery (LAD) (Figure 1) (Video 1).
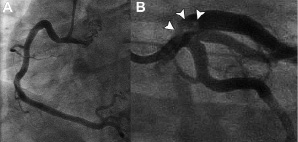
Figure 1. A: Normal appearance of the right coronary artery. B: Large filling defect (arrows) in the left main extending into the proximal left anterior descending artery.
The angiographic appearance of left circumflex artery (LCX), large intermediate branch (IB) and dominant right coronary artery (RCA) was unremarkable with normal epicardial flow, but the flow in the LAD was mildly impaired (TIMI flow, grade 2) (Video 2).
At this stage, it was unclear what would be the optimal treatment strategy. It was deemed too high risk to performing a PCI due to the location of the thrombus and the complexity of the three major epicardial vessels. Any attempt of thrombotic aspiration had the risk of possible embolisation towards the three distal run-off vessels. An early consultation with our surgical colleagues suggested that ideally, coronary artery bypass graft surgery (CABG) would carry the least risk, but preferably not in the acute phase of a MI.
Since the patient was now completely pain free following catheterisation and there was spontaneous reperfusion of the infarct-related-vessel(s), it was decided for aggressive anticoagulant and anti-platelet therapy. On a regime of aspirin, clopidogrel, unfractionated heparin, and IV eptifibatide, a glycoprotein IIb/ IIIa receptor antagonist, the patient’s clinical condition stabilised. The CK values peaked at 454 U/l (CKMB mass of 47.6 U/l) 16 hours after admission.
But one day after admission she developed a spontaneous bleeding of the right iliopsoas muscle accompanied by fall in haemoglobin level from 6 to 4.3 mmol/l. The treatment with eptifibatide was stopped and she was transfused two units of packed red blood cells. On selective abdominal angiography there was no obvious bleeding focus.
The patient underwent a CT coronary angiography (CTCA) three days later to re-evaluate the left main stem and other coronary arteries. The CT scan showed a persistent severe narrowing of the LMCA with normal appearance of the other coronary vessels (Figure 2).
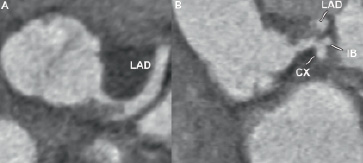
Figure 2. Axial (A) and coronal (B) CT image showing the thrombus in the LMCA. The thrombus extends towards the LAD. The IB and CX
are not affected. CX: circumflex artery; IB: intermediate branch; LAD: left anterior descending artery; LMCA: left main coronary artery.
Assessment of the total coronary calcium burden did not reveal any calcium. The invasive coronary angiogram that was repeated the same day confirmed these findings. (Video 3) By this stage, the CK level had normalised (134 U/l).
How could I treat?
The Invited Experts opinion
Despite the lack of the typical signs of fat embolism syndrome, the hypothesis of fat embolism in a young woman with sickle cell disease as the cause of an acute myocardial infarction with persistent non-occlusive thrombosis of the left main should be considered. Again, there is no angiographic evidence of atherosclerosis or spontaneous coronary dissection as the cause of thrombosis. It could be useful to see the distal bed of the left anterior descending artery, since ST segment elevation in both precordial and inferior leads associated with a subsequent small infarct suggest a persistent occlusive embolism of the distal left anterior descending artery. It has been hypothesised that fat emboli may reach the systemic arterial circulation via a patent foramen ovale or other intracardiac or pulmonary arteriovenous shunts1 and appropriate investigations to identify these pathologies should be performed (ECO, CT scan). The lack of any visible effect after three days of aggressive antiplatelet and anticoagulant treatment makes stronger the suspicion that the mass in the left main cannot be a thrombus.
Another possible role of sickle cell disease in this case is that the thrombosis may be the result of endothelial damage attributable to shear injury by sickle cells and resulting prothrombotic state.
At this stage, my strategy would be to perform an IVUS examination in order to exclude the possibility of coronary atherosclerotic disease or spontaneous dissection complicated by thrombosis. Again, there is the possibility that IVUS virtual histology could define the characteristics of the mass going into the left main.
In the case of a fat embolism diagnosis, I think that the best strategy is the surgical removal of the material, since all endoluminal techniques (thrombectomy devices, atherectomy devices, aspiration devices also with large catheters such as the Proxis used as an aspiration catheter without proximal occlusion of the vessel) seem likely to be unsuccessful in removal the entire material, while the risk of distal occlusive embolisation is high. Conversely, if the mass is a thrombus, the treatment should be endoluminal and should include rheolytic thrombectomy before stenting, or thrombectomy alone if IVUS doesn’t show any disrupted plaque or dissection.
Whatever the nature of the mass going into the left main and the chosen treatment (endoluminal or surgical treatment), in my opinion there is the indication for acute exchange transfusion before treatment in order to decrease the haemoglobin S level to less than 30%. It has been shown that hyper-transfusion may prevent sickling and decrease the pro-coagulant state of sickle cell disease2.
This is a particularly interesting and unusual case of left main stem (LMS) stenosis. The optimal method of revascularisation (between surgery and stents) will be influenced by how general recommendations for LMS stenosis are influenced by key features specific to this patient including
– Young woman (43 years old)
– Presentation with established acute infarct with ST elevation and enzyme rise
– Complex left main stem stenosis involving ostium, shaft and extending to proximal LAD and containing thrombus (may require IVUS confirmation)
– Normal intermediate, obtuse marginal and right coronary artery
– Significant bleed on anti-platelet medication
Additional information required to determine optimal treatment decision
– Precise extent and complexity of LMS stenosis can be delineated by IVUS
– Assessment of left ventricular function (good, moderate, poor) to guide risk of surgery
– Any other contraindication to surgery such as significantly impaired renal function
The case for and against stents
In general outcomes with stents in LMS stenosis are critically dependent on
– the precise anatomic location and complexity (including calcification) of the lesion
– the simultaneous presence of multivessel coronary artery disease (in up to 80% of patients).
Up to 90% of LMS stenosis involve the distal segment of the artery and extend into the proximal coronary arteries1 and such bifurcation or trifurcation lesions are at notoriously high risk of restenosis with both bare metal stents and drug eluting stents2. The angiographic appearance in this patient suggests diffuse involvement of the left main including extension to the LAD (if there is uncertainty this can be confirmed with IVUS). Furthermore -and ominously- restenosis in this critical location, even with drug eluting stents, is frequently asymptomatic and therefore mandates intensive and possibly serial angiographic follow up3.
In contrast, a recent multicentre retrospective registry of 147 patients with non-bifurcation LMS appears to be safe and effective with restenosis of <1% at 6-month angiographic follow-up, major adverse clinical event rates of 7%, and a cumulative cardiac mortality of 2.7% at a median follow-up of around two and a half years4. However even in this series the authors pointed out that there were four late unexplained deaths4 raising the spectre of late stent thrombosis.
If stenting is advocated then the relative merits of drug eluting (DES) and bare metal stents (BMS) need to be considered. While DES reduce the rate of restenosis it is increasingly likely that they will require lifelong dual anti platelet medication. In view of a previous significant bleed in this patient DES may be contraindicated.
The case for and against CABG
Both randomised trials and Registry data show that CABG offers the most durable and proven treatment for LMS stenosis1. In contrast to the location of stents, placing the bypass grafts to the mid coronary vasculature has two important beneficial prognostic implications1:
– the complexity and location of LMS stenosis (as well as its frequently associated proximal multivessel coronary artery disease) are irrelevant
– the grafts offer prophylaxis against the development of new disease in the proximal coronary vasculature
And the current results of CABG in LMS stenosis are reassuring. The Society of Cardiothoracic Surgery in the UK reported a mortality of 3% for all 5,003 patients undergoing CABG for LMS stenosis including high risk and urgent cases5 and two recent British studies reported excellent two year survival rates of 94% and 95%6,7. As for all cardiac surgical procedures the mortality is strongly influenced by co-morbidities; for example Ellis et al reported that three year mortality for CABG in LMS stenosis varied from 4% in low risk patients to 40% in those with significant and multiple co-morbidities8. The fact that this patient has had a recent infarct would significantly increase the immediate risk of surgery with the risk decreasing rapidly after two weeks and towards normal elective risk within six weeks assuming that the ventricle is not significantly impaired.
The survival benefit of CABG is increased by the use of an IMA graft to the left anterior descending coronary artery and may be further magnified by the use of a second IMA graft. The angiographic patency of both IMA placed to left sided coronary vessels is over 95% at one week and at seven years and may translate into a significant survival benefit9. In a systematic review of over 15,000 CABG patients matched for age, gender, diabetes and left ventricular function, the HR for death was 0.82 with bilateral vs single IMA grafts (NNT of 13-16)9. And the use of bilateral IMA grafts appears safe; the ART trial of bilateral versus single IMA10 grafts reported an overall 30-day mortality of 1% in over 3,100 patients [Taggart DP; unpublished observations].
Recommendation
If the patient remains stable, she should be kept in hospital to undergo CABG in 10-14 days (assuming LV function is at least moderate) as this offers the best prospect of a good long-term outcome and particularly in view of her young age. An initial strategy of stenting, to defer surgery, carries risk because of an increased risk of death before crossover to CABG occurs. CABG should be performed with three bypass grafts because of the diffuse nature of LMS disease and ideally should be done with both IMA grafts to the two best left sided vessels. If the patient is unstable or has sustained significant myocardial damage (when risks of CABG would be high) then an initial strategy of stenting is reasonable but mandates intensive – including angiographic – follow-up. In view of previous significant bleed then a bare metal stent might be favoured to avoid the need for dual antiplatelet medication.
How did I treat?
Actual treatment and management of the case
The patient was deemed eligible for enrolment in the SYNTAX study, a multicentre, prospective study, randomising patients with de novo 3-vessel disease and/or left main disease to undergo either PCI or CABG. She was randomised to the CABG-arm of the study and eight days after admission she underwent uneventful revascularisation of the LAD using the left internal mammary artery graft, and of the intermediate branch using the right internal mammary artery graft. A small aortotomy was performed to remove the focal lesion in the LMCA, which was sent for pathologic evaluation (Figure 3).
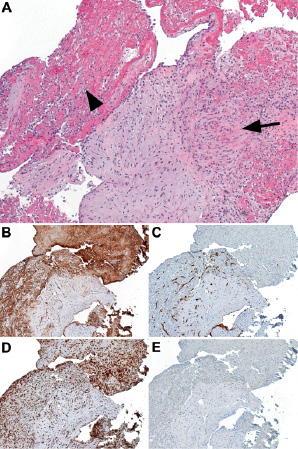
Figure 3. A: Tangential cut of fibrotic intima with attached thrombus that is being organised in view of the ingrowth of fibroblasts (arrow) and capillaries (arrowhead) (hematoxylin and eosine, original magnification 50 x). B: Same area stained for CD31 shows diffuse staining of thrombotic material including platelets and capillaries. C: Same area stained for CD34, highlighting the growth of capillaries into the thrombus. D: Same area stained for CD68, showing numerous histiocytic cells/macrophages scattered throughout the intima and the thrombus. E: Same area stained for desmin showing the absence of smooth muscle cells in the intima.
She made an uneventful recovery and was discharged eight days later on 100 mg aspirin, 100 mg metoprolol, 40 mg atorvastatin, 4 mg perindopril once daily, and oral iron supplements. To re-assess the surgical result, a repeat cardiac CT scan was performed six months later, which showed a patent LMCA without residual stenosis and occlusion of both bypass grafts (Figure 4a).

Figure 4. A: Three-dimensional volume-rendered CT image showing the occlusion of the LIMA (left internal mammary artery graft) [arrowhead] at the level of the right ventricular outflow tract. The RIMA (right internal mammary artery graft) is also occluded: its previous course is still marked by vascular clips (arrows). B, C: Corresponding invasive angiogram showing the LIMA graft to be hypoplastic with only minimal contrast filling distally (arrowheads) and an occlusion of the RIMA in its proximal part (arrow). D: Invasive coronary angiogram showing a normal appearance of the left main coronary artery (arrowheads).
A repeat invasive angiogram showed the LIMA to be hypoplastic with competitive filling from the native coronary circulation (Figure 4b); the RIMA graft was occluded early on in its course (Figure 4c).
Intravascular ultrasound (IVUS) showed the persistence of a large noncalcified plaque in the LMCA, that was clearly present on CT but not visible angiographically (Figures 5a and b). I

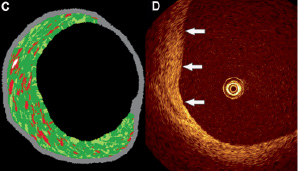
Figure 5. A: Intravascular ultrasound of the distal left main coronary artery showing a large noncalcified plaque, extending from the 6 to 12 o’clock position. B: This plaque is also visible on the corresponding CT image (arrows). The previous connection of the right internal mammary artery graft to the intermediate branch is indicated by the vascular clip (arrowhead). C: Radiofrequency data analysis (RDA) showed that the plaque was predominantly fibrous in nature. D: Optical coherence tomography (OCT) also showed features consistent with a fibrous plaque with a high reflectivity, homogeneous and finely textured appearance (arrows).
VUS radiofrequency data analysis (RDA) and optical coherence tomography (OCT) showed this plaque to have a fibrous composition (Figures 5c and d). Serology revealed that the patient was heterozygous for the haemoglobin S gene without any other pro-thrombophilic condition. An overview of the relevant laboratory tests performed since hospital admission is provided in Table 1.
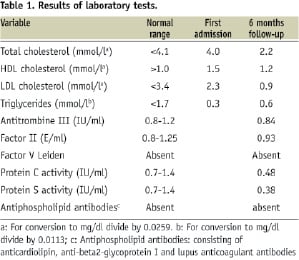
Since she remains asymptomatic, her medical therapy was continued.
Discussion of diagnosis
The occurrence of an acute MI in a female patient of relatively young age, i.e., less than 45 years, is quite unusual and represents only ~ 2% of the women diagnosed with a heart attack each year.1 Except for arterial hypertension and a positive family history of premature coronary artery disease our patient did not have other risk factors that would put her at risk for developing an acute MI. Using the Framingham-risk score her 10-year risk for developing a major coronary event was calculated as less than 1%.2 Illicit drug use, which our patient denied, is a well recognised precipitating factor in this age group and should be scrutinised. Coronary calcium, as measured and quantified by cardiac CT, documents the presence of coronary atherosclerosis and adds precision to the risk estimate of cardiac events based on traditional risk factors.3 The risk of cardiac events in patients without coronary calcification is very low, i.e., 0.1% per year.4,5 However, the absence of coronary calcium does not exclude the presence of atherosclerotic disease. In fact, it is not unusual to find a calcium score of zero in relatively young patients (i.e. below the age of 60) who present with an acute coronary event.6 Plaque rupture and ensuing thrombosis most often occurs in non-calcified coronary lesions. CTCA allows the identification of these so-called soft plaques provided they are sufficiently large. This case illustrates this concept and is a nice in vivo illustration of plaque erosion with thrombosis occurring in an atherosclerotic lesion with non-calcified morphology. Coronary plaque erosion is a less frequent cause of coronary thrombosis and typically occurs in relatively young and female patients.7 As compared to the classical rupture-prone plaques, this type of vulnerable plaque typically lacks a large lipid core but instead is composed of smooth muscle cells and proteoglycan-rich extracellular matrix.
Patients with sickle cell disease, who are homozygous for the abnormal haemoglobin S, can present with cardiac problems, including the occurrence of acute MI.8 In contrast to atherosclerotic disease, coronary thrombosis with obstruction usually affects the small arteries and it is rare to find abnormalities of the large epicardial vessels. Furthermore, heterozygosity for the sickle cell gene (sickle cell trait) usually does not result in clinical manifestations. Although the pathogenesis of the acute cardiac event in this case is related to atherosclerotic disease, the abundance of endothelial cells within the removed thrombus-like material in the left main stem, fits with the histopathologic findings of patients with sickle cell disease.9 Factors such as endothelial dysfunction and increased platelet activation, which are well described in patients with sickle cell disease, might have contributed to the sequence of events in this patient.10
Current 64-slice and dual-source 64-slice CT scanners offer sufficient resolution to provide reliable non-invasive access to the different cardiac anatomical structures. Of particular value is the direct way of looking at coronary artery disease by revealing the presence of disease in the vessel wall and lumen and the robustness in visualising larger vessel structures such as bypass grafts, which sometimes are difficult to depict by invasive coronary angiography. The cardiac CT scan in this case accurately reflected the course of the disease by showing the presence and subsequent disappearance of the large plaque with luminal narrowing in the LMCA and the occlusion of the bypass grafts at six months follow-up.
Treatment and management
Primary PCI using stents performed within 90 minutes of first medical contact is the preferred treatment strategy for patients with STEMI who present within 12 hours of symptom onset.11 However, up to 5% of patients with STEMI who undergo emergency cardiac catheterisation are not deemed suitable for PCI but undergo CABG instead.12 The main reason to select CABG as the preferred reperfusion strategy in patients with acute MI is the presence of left main disease or other unfavourable anatomy for PCI. In selected patients, in particular acute MI patients with an occlusion of the left main stem and/or haemodynamic instability, the percutaneous approach is often pursued. In the current case, spontaneous reperfusion of the infarct-related artery had occurred, which gave the opportunity to reconsider the different therapeutic options. The presence of unprotected left main disease is considered a surgical indication, thus surgery would have been the preferred therapeutic approach.13 However, several advancements in the field of interventional cardiology, such as the advent of drug-eluting stents (DES), have improved the outcome of patients with more complex coronary disease and are challenging the results of CABG. Large randomised trials currently underway comparing PCI using DES with CABG for LMCA disease such as the SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study and the COMparison of Bypass surgery and AngioplasTy using SES in patients with LMCA disease (COMBAT) study, will better define the role of PCI in this particular group of patients.14
When CABG is deemed necessary in a patient with STEMI, the timing of surgery becomes important. Recent studies have demonstrated that, as for PCI, CABG is preferably performed within six hours of symptom onset or alternatively to delay surgery for at least 24 hours and preferentially seven days. In-hospital mortality becomes excessively high within the six to 24 hour time interval and there is general consensus to avoid this critical period whenever possible.15
The third option would have been to perform an isolated percutaneous or surgical resection of the thrombotic material. Percutaneous thrombectomy has not been shown to be effective in randomised studies, although there might be a niche application for this practice in lesions with a large thrombus load.16 In this case, the thrombotic lesion was removed surgically in combination with the implantation of two bypass grafts. As documented by the CT scan and confirmed angiographically, both grafts became functionally and/or anatomically occluded. This is not an unexpected finding since it is well documented that bypass grafts often occlude when they are attached to native coronary arteries with only mild or moderate stenoses.17
Conclusion
Primary PCI is advocated as the preferred therapy for patients with STEMI. However, a patient-specific approach is necessary and cardiac surgery remains an appropriate choice in patients with unfavourable coronary lesion anatomy. In the latter situation, bypass grafts are not always needed and become unnecessary in the case of non-flow limiting coronary lesions.
This case furthermore demonstrates the possibilities of cardiac CT as a non-invasive imaging tool to visualise disease-related issues of native coronary arteries and bypass grafts.
Online data supplement
Video 1. Angiogram RCA
Video 2. Angiogram LCA baseline
Video 3. Angiogram LCA follow-up
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.

