Description
The Medical Positioning System (MPS) is based on magnetic tracking technology and is employed in conjunction with an X-ray imaging system in a catheterisation laboratory. By using the system, MPS-enabled devices can be localised, tracked, and navigated. A sub-millimeter sensor assembled on the device’s tip senses the low-intensity magnetic field generated by the MPS, and generates an electrical signal by which accurate real-time position and orientation (P&O) information is computed. The magnetic field transmitters, located around the flat detector, are synchronised with the operation of the flat image detector to avoid X-ray image distortion. A magnetic reference sensor (ECG electrode-size) is attached to the patient’s chest to provide information about the spatial relationship between the chest wall and the flat image detector. This magnetic chest-wall sensor is also used for assessment of respiratory movements. The inherent co-registration of the MPS information with the X-ray based imaging, allows for accurate display of the device’s P&O on live fluoroscopy and pre-recorded cine-angiography. A 3D image modality displays the vessel anatomy and the device’s location.
The MPS core system capabilities, available with any MPS-enabled device are:
– Continuous real-time tracking of the device tip projected on:
- live fluoroscopy
- pre-recorded, cine-angiography (with no further need for fluoroscopy or dye)
- 3D vessel reconstructed images.
– Automatic 3D model reconstruction of the arterial lumen, generated from angiographic image segmentation and the MPS-based 3D spatial information of the MPS-enabled devices.
– Assessment of the vessel’s foreshortening using a colour-coded trace (Smart Trace™) superimposed onto either live X-ray images or cine-loops.
– 3D length measurements of a selected segment, based on the trace of the MPS-enabled device.
– Virtual landmarks (VLM) of points or segments of interest which are superimposed on both 2D (X-ray image) and 3D reconstructions.
When an MPS-enabled device is introduced into the inspected vessel, the MPS system collects its tip’s spatial P&O and records the patient’s real-time respiration and heart rate. Currently the procedure sequence is initiated by a slow pullback of the MPS enabled device from the distal vessel to the guiding catheter. Data acquired during pullback are used to analyse the respiratory and cardiac motion patterns. The information is processed to generate the “Smart Trace™” that can in turn be projected onto live or cine-images. The Smart Trace™ refers to a coding of the centreline-3D vessel structure foreshortening projected on a 2D image using colour-coding. Cold colours denote minimal or no foreshortening while hot colours denote significant foreshortening (more than 10%).
Integration of the Smart Trace™ with the information obtained from segmentation of the angiographic images is used to reconstruct an accurate 3D model of the studied vessel. The 3D model, shown on a MPS dedicated display monitor, can be manually manipulated to provide better understanding of vessel anatomy.
Using both heart and respiration rate and pattern to compensate for relative motion, the real-time catheter tip P&O can be projected onto previously acquired cine-loops. This unique feature of the MPS system is possible using proprietary algorithms that allow instantaneous overlay of current catheter tip position onto previously acquired cine-loops. This algorithm corrects for differences in heart rate and chest wall motion between the pre-recorded cine-loops and current device position.
Landmarks may be placed by the physician on anatomic points of interest either by using a cine-loop or the 3D model. Once placed, the landmarks can be continuously projected onto live angiography. Simultaneous display of the live position and projection onto a cine-loop may be used to generate a bi-plane like image.
The MPS and the Guided Measurement Catheter (GMC™), a diagnostic catheter used during cardiac catheterisation obtained the CE Mark of Conformity, no. 0344, in 2007, and are commercially available.
The following MPS-enabled prototypes were tested in the laboratory and animal experiments:
GIVUS – Guided intravascular ultrasound – collects ultrasound images with their corresponding spatial information. GIVUS uses a near real-time segmentation algorithm to automatically identify the vessel lumen and media to generate an accurate 3D model of the vessel.1
Guided PHV delivery system – a delivery system consisting of a percutaneous heart valve (PHV) equipped with an MPS sensor. The device provides anatomical landmarks based on previously acquired images and enables accurate valve delivery and placement.
Guided stent – a stent delivery system equipped with an MPS sensor, enables stent delivery at the pre-determined segments marked by virtual landmarks on pre-recorded cine-loops, still angiographic images, or 3D reconstructed vessels. Its use is expected to reduce radiation dose and use of contrast media.
Guided guidewire – a PCI guide wire equipped with an MPS sensor at its tip. The orientation and position of the wire’s tip are continuously projected onto live fluoroscopy or cine-angiography. Its use is expected to enable accurate guide wire navigation and reduced dosage of radiation and contrast media.
Technical specifications
Guided measurement catheter specification
The GMC™ is a rapid-exchange catheter, with an MPS sensor at its distal tip. The sensor is radio-opaque and replaces the distal radio-opaque marker. The GMC™ is connected to the MPS system using an electric connector, as shown in Figure 1.
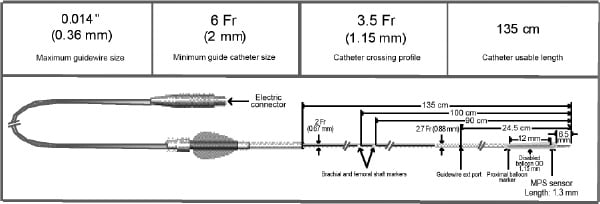
Figure 1. GMC™
MPS system specification
The MPS system is equipped on Siemens AXIOM Artis dFC and Philips Allura FD10. Its components are (see Figure 2):
a. Magnetic transmitters attached to the flat detector
b. Two additional monitors
c. Table-side controls for system operation
d. MPS console
e. Patient sensor
Figure 2. MPS System installed in a catheterisation laboratory
INDICATIONS FOR USE
The MPS equipped on an X-ray imaging cath lab system is intended for intravascular evaluation of coronary anatomy. It is intended to enable real-time positioning and navigation of an MPS enabled (equipped with an MPS sensor) diagnostic or therapeutic invasive device used in coronary or cardiac intervention in the cath lab environment, on both live fluoroscopy or recorded background.
The MPS enabled GMC™ intravascular device is intended to be used in conjunction with conventional X-ray angiography systems equipped with MPS, to enable real-time tip positioning and navigation, quantitative length measurement, 3D lumen reconstruction, qualitative 3D foreshortening indication and landmarking, in patients who are candidates for coronary angiography and/or percutaneous coronary.
PRECLINICAL AND INITIAL CLINICAL EXPERIENCE
The performance, safety and feasibility of the MPS technology and of various MPS enabled device prototypes were tested in animal studies (50-60kg male domestic swine).
MPS Guided IVUS, MPS guided percutaneous heart valve, MPS ready stents, and GMC were tested in a set of animal experiments showing safety and implying the clinical potential of these devices.
Based on animal studies findings, a prospective, single arm, single-centre, non-randomised, open label study (MPS in Conjunction with the GMC™ – Feasibility Study in Clinical Routine) was conducted at the University Hospital, Regensburg, Germany (Investigators: A. Jeron MD and A. Luchner MD). In this study, AXIOM ARTIS dFC imaging system and MPS were integrated in collaboration with Siemens Healthcare Sector. Twenty patients were enrolled and underwent the MPS-GMC™ procedure prior to PCI. Following coronary angiography, the GMC™ catheter was introduced via a 6 Fr guiding catheter, over a 0.014’’ guidewire to distal index coronary artery and then pulled back (Figures 3 and 4).
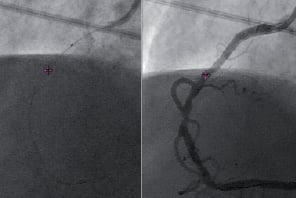
Figure 3. GMC Tip Tracking onto Live (left) and Cine (right)
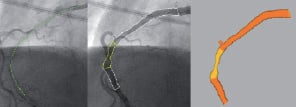
Figure 4. Smart Trace™ (left), 3D model projected on Cine (middle) and 3D model (right)
The GMC™ device has reached all performance primary endpoints without any device and/or procedure related adverse events in all 20 patients.
Conclusion
The MPS in conjunction with MPS enabled devices is a novel technology with potential to improve the quality of percutaneous interventions. The first clinical trial with a GMC™ was completed at the end of 2007 demonstrating its safety and reaching the performance endpoints. Further work will be performed to prove the efficacy and added value of this technology. The main advantages of the MPS enabled imaging in the coronary arena are the ability to truly measure the 3D length, perform 3D QCA and to measure and quantify foreshortening.
The effects of use of the MPS imaging on reduction of X-ray exposure and use of contrast media will be investigated. The use of this technique alone or in conjunction with multi-detector CT imaging for interventions in chronic total occlusions will be also explored.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.
Moving image 4.
Moving image 5.

