Description
Kaneka’s coronary stent, MAHOROBA™, is a new drug eluting stent (DES), which combines a thin, flexible cobalt-chromium (CoCr) metal stent platform coated with a biodegradable polymer including the pharmaceutical agent tacrolimus. The device has been designed and developed to inhibit the growth of neointimal hyperplasia and avoid interference with the vascular healing response.
History
The advent of DES has revolutionised the practice of interventional cardiology by inhibiting the development of neointimal hyperplasia and thereby reducing the rates of restenosis and repeat revascularisation as compared to bare metal stents (BMS)1-4. After the first approval of DES in 2002, a large number of patients with coronary artery disease have undergone percutaneous revascularisation with DES. Recently, however, certain potential safety concerns regarding their widespread use have arisen. The most notable drawback of DES is that they could increase the risk of thrombotic complications, especially late stent thrombosis. In order to discuss this serious problem, the US Food and Drug Administration held a medical device advisory panel meeting on December 7-8, 20065. They concluded that DES are associated with a numerical excess of late stent thrombosis (after 1-year post implantation), although the magnitude of this excess is uncertain.
The increased risk of thrombosis with the use of DES may be associated with altered endothelial function6 and/or delayed vascular healing7 induced by cytotoxic and cytostatic drug use. In addition, localised hypersensitivity reactions to the polymer coating of the DES and drug itself may also contribute to stent thrombosis8,9. To retain the positive clinical aspects of DES and overcome their drawbacks, Kaneka Corporation has developed a new DES, MAHOROBA™, which aims
to inhibit excessive neointimal growth whilst avoiding the interruption of the vascular healing process, including re-endothelialisation.
Stent characteristics
1. PLATFORM
Material: cobalt chromium (CoCr) alloy
Deployment: balloon expandable
Strut thickness: 75 µm (0.0030 inches)
Size available: 3.0 x 18 mm and 3.5 x 18 mm
Number of cells: both 8 cells and 10 cells are combined
Number of joints: 2
Radial force: 0.17 N/mm (for the 3.0 mm diameter stent)
Recoil/shortening: 3%/3%
Nominal/rated burst pressure: 9 atm/16 atm
2. DRUG
Used drug: tacrolimus
3. COATING
Characteristics: biodegradable polymer
Composition: poly DL-lactide-co-glycolide (PLGA: 3.58 µg/mm2)
+ tacrolimus (0.94 µg/mm2)
Coating methods: whole abluminal surface coating of the stent platform
Drug release kinetics: controlled with half the drug released over more than 84 days
4. DELIVERY SYSTEM
Guide compatibility: 6 Fr
Outer diameter: 2.7/2.1 Fr (in the distal/proximal shaft)
Technical specifications
1. PLATFORM
The material of the stent platform is CoCr. The stent has an open-cellular balloon-expandable design and consists of two helical coils inter-crossed with two phase-different links on each turn, in which each link deviates diagonally along the longitudinal axis (Figure 1).
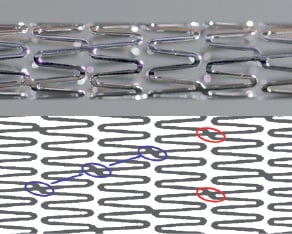
Figure 1. MAHOROBA™ Tacrolimus eluting coronary stent.
The upper part of this figure is a magnified image of the MAHOROBA™ tacrolimus eluting coronary stent. The lower is a schematic of the stent structure itself. Two helical coils inter-cross with two phase-different links (red circle). Blue circles and arrows indicate that each link deviates diagonally along the longitudinal axis. These novel configurations of links achieve high flexibility and vessel scaffolding.
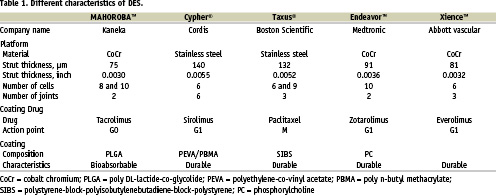
This novel link configuration allows for low elastic recoil, sufficient radial force and good scaffolding while featuring excellent flexibility both mounted on the delivery balloon as well as after expansion. In addition, the stent strut thickness (75 µm, 0.0030 inch) of the MAHOROBA™ stent is thinner than most other DES (Table 1).
Randomised clinical trials have revealed that thin-strut BMS have significantly reduced restenosis rates as compared to thick-strut BMS10, probably because thin-strut stents cause less arterial injury at deployment. Therefore, the MAHOROBA™ stent may reduce the growth of neointimal hyperplasia more than the other DES.
2. DRUG
Tacrolimus (FK 506: Astellas Pharma Inc., Tokyo, Japan) is a water-insoluble macrolide immunosuppressant indicated for the prophylaxis of organ rejection in patients receiving allogeneic liver or kidney transplantation. Tacrolimus binds to FK-binding protein12 (FKBP12), forming a compound (Tacrolimus-FKBP12 complex), which inhibits the production of pro-inflammatory cytokines such as interleukin 2 by preventing the activation of calcineurin and suppresses T-cell proliferation (immunosuppressive effect)11. This complex also inhibits several steps of the cascade of events leading to neointimal formation12 and reduces the proliferation of smooth muscle cells (SMC)13, which play an important role in the mechanism of restenosis (antiproliferative effect). These two effects of tacrolimus contribute to the inhibition of neointimal growth.
Unlike drugs used in other DES, tacrolimus has a different mode of action for the cell cycle (Table 1). By reducing the expression of cell cycle proteins, tacrolimus holds cells in the G0 phase, in which cells are able to function, but unable to replicate. Sirolimus, zotarolimus and everolimus arrest cell replication in the G1 phase (cytostatic effect), whilst paclitaxel prevents mitosis and then halts the metaphase (cytotoxic effect). Therefore, tacrolimus is a non-cytotoxic and non-cytostatic agent, which may be beneficial in preventing excessive vessel injury.
Additionally, tacrolimus is also expected to demonstrate less inhibition of endothelial cell (EC) proliferation, which is an important process for vascular healing. Matter et al reported in an in vitro study that the inhibitory concentration50 (IC50) value of tacrolimus for EC is markedly higher than that for SMC, while the IC50 value of sirolimus for EC is remarkably lower than that for SMC14 (Figure 2).
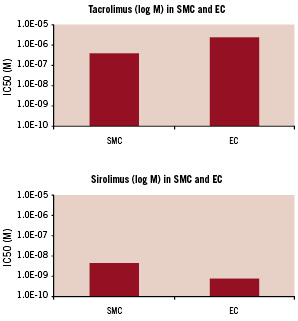
Figure 2. Inhibitory concentration50 (IC50) values of tacrolimus and sirolimus for smooth muscle cells and endothelial cells.
The upper panel shows the inhibitory concentration50 (IC50) values of tacrolimus for smooth muscle cells (SMC) and endothelial cells (EC). The bottom panel shows the IC50 values of sirolimus for SMC and EC. The IC50 value of tacrolimus for EC is markedly higher than that for SMC, while the IC50 value of sirolimus for EC is remarkably lower than that for SMC. Data are extracted from Matter et al14.
This result implies that if equipotent concentrations for suppressing SMC proliferation are used, tacrolimus may allow better re-endothelialisation than sirolimus. Moreover, unlike sirolimus and paclitaxel, tacrolimus does not affect tissue factor expression, which can lead to the initiation of coagulation causing thrombus formation15,16. Tacrolimus may therefore reduce the incidence rates of stent thrombosis as compared to the other drugs.
Considering these characteristics, if effective local drug concentrations are achieved, tacrolimus can exert its antiproliferative and anti-inflammatory effects while being vascular protective with less prothrombotic effects. Therefore, tacrolimus is a promising compound for the next generation of DES.
3. COATING POLYMER AND DRUG RELEASE KINETICS
The whole abluminal surface of the stent platform is coated with a matrix containing bioabsorbable polymer and tacrolimus. The surface of the coating layer is generally smooth and no cracks or peeling are observed even when the stent expands (Figure 3).

Figure 3. Surface of MAHOROBA™ stent.
Scanning electron microscopy images show that the surface of the expanded stent is smooth (left panel). Even in the magnified images (right panel), no cracks or peeling are observed.
Most other DES are coated with a durable polymer as a drug carrier (Table 1). A durable polymer may lead to hypersensitivity with eosinophilic infiltration in surrounding tissues at the stent deployment site17 since the polymer exists permanently. This hypersensitivity reaction could be associated with stent thrombosis8. To minimise this undesirable reaction, biodegradable materials can be an alternative vehicle for drug delivery because they are fully metabolised into carbon dioxide and water18, and thus no chemical substances remain permanently. Kaneka Corporation has developed a specially designed fully biodegradable polymer composed of PLGA to achieve a long degradation time. In a porcine model, the PLGA completely disappears from the coronary artery in approximately 6 months (Figure 4).
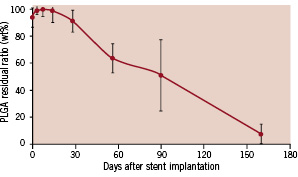
Figure 4. Residual ratio of PLGA after stent implantation in a porcine coronary artery. The PLGA degrades gradually and completely disappears in approximately 6 months.
As the drug is uniformly distributed in the polymer layer, tacrolimus will be released continually for several months and completely disappear concomitantly with PLGA degradation. Indeed, in an in vivo porcine coronary model, the tacrolimus release kinetics of the MAHOROBA™ stent are controlled with 26% and 45% of the drug released in 28 days and 84 days, respectively, while the sirolimus-eluting stents (Cypher®: Cordis Corporation, Warren, NJ, USA) are designed such that 79% of the sirolimus has been released by 28 days19. This sustained drug release property results in subsequent retention of a sufficient concentration of tacrolimus in target coronary arterial tissues for several months (Figure 5).
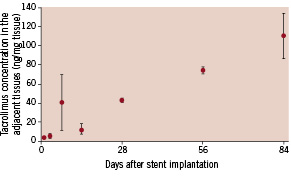
Figure 5. Tacrolimus concentration in the adjacent arterial tissues. Drug concentration in the surrounding tissues gradually increases, resulting in the retention of a sufficient concentration of tacrolimus in target coronary vessels for several months.
The coating method of the MAHOROBA™ is completely different from a previously investigated tacrolimus-eluting stent (Janus™, Sorin Biomedica Cardio s.r.l., Saluggia, Italy)20. The Janus™ stent does not have any polymer vehicle, but has deep reservoirs containing tacrolimus on the external abluminal stent surface, in which controlled drug release is difficult. Consequently, the vessel wall may acutely be exposed to extremely high concentration of the drug, leading to excessive inflammation in vascular walls. An animal study showed that the tacrolimus concentration in the arterial wall peaked a few days after the Janus™ stent implantation and then steeply fell to steady values12. These release kinetics may be a part of the reason why the Janus™ stent had a neutral effect on restenosis (in-stent late luminal loss of 0.65 mm) as shown in the Jupiter II trial20. Although the same drug is used, the MAHOROBA™ stent may prevent more neointimal hyperplasia than the Janus™ stent, because the MAHOROBA™ stent features controlled drug release.
4. DELIVERY SYSTEM
The delivery system is based on the rapid exchange type PTCA balloon catheter (Fortis™, developed by Kaneka Corporation) with a semi-low-compliant balloon. This device is approved and has been sold in Japan.
Preclinical experience
Preclinical testing started in 2005 and is ongoing into the second quarter of 2007. The initial animal results were presented during EuroPCR 200621. It showed that the MAHOROBA™ reduced neointimal thickening and neointimal area at 1 and 3 months following stent deployment as compared to BMS, while it allowed for vascular healing and endothelialisation. Kaneka in-house porcine data also showed that well endothelialised neointima was visible as early as 14 days (Figure 6) after stenting without visible qualitative differences between MAHOROBA™ and BMS.
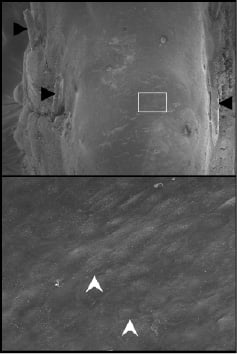
Figure 6. Representative scanning electron microscopy images from MAHOROBA™ at low and high power magnification.
MAHOROBA™ shows almost complete re-endothelialisation 14 days after deployment (upper panel: low magnification). Stent struts are almost completely covered by the endothelial cells and embedded in the arterial wall. Black arrowheads indicate stent struts. Bottom panel is a magnified image of the white box in the upper panel. Endothelial junctions (white arrow head) are clearly visible.
The biodegradable PLGA polymer did not cause obvious luminal inflammation.
Future directions
In summary, the principal characteristics of MAHOROBA™ are the following. First, the coating drug, tacrolimus, is a non-cytotoxic and non-cytostatic drug. Tacrolimus prevents the growth of neointimal hyperplasia and allows for better re-endothelialisation. Second, the coating polymer is fully absorbable and minimises adverse effects caused by the permanent presence of polymer. Third, the specially designed biodegradable polymer can sustain longer drug release, which allows a sufficient concentration of tacrolimus in coronary arterial tissues for several months. Lastly, the stent strut thickness is small, which may also contribute to reducing neointimal growth.
The primary goal of the next generation of DES is no longer the complete inhibition of neointimal hyperplasia but the restitution of a healthy, functionally active endothelial lining capable of modulating the healing process, in order to prevent potential drawbacks of DES, such as stent thrombosis. The MAHOROBA™ is a second generation of DES, which aims to address these issues. To evaluate the safety and efficacy of the MAHOROBA™ stent in humans, the first-in-man study, which is a prospective, single-armed, multicentre, core lab analysis study, will be launched in May 2007.
Study name: MAHOROBA I
Study P.I.: Patrick W. Serruys
Enrolment: 45 patients
Primary endpoint: In-stent late luminal loss at 4 and 12 months
Clinical site: 3 clinical sites in The NetherlandsThoraxcenter/ Rotterdam (P.W. Serruys, P.I.)
Medisch centrum Rijnmond-Zuid/Rotterdam (P. Smits, P.I.); Amphia Ziekenhuis/ Breda (P. den Heijer, P.I.)
Core Lab: Cardialysis/ Rotterdam, The Netherlands (Angio/ IVUS/ OCT/ MSCT)

