Abstract
Aims: Comparison of magnetic guidewire navigation in percutaneous coronary intervention (magnetic PCI) across distal and/or complex lesions versus conventional navigation (conventional PCI).
Methods and results: Forty-seven consecutive patients (age 61±10yr) undergoing elective single vessel magnetic PCI for distal and/or complex lesions were matched by age and lesion location with 45 patients undergoing conventional PCI (age 63±10yr). Technical success rate was defined as an intraluminal wire position distal to the stenosis. Procedural outcome and costs were evaluated. Baseline demographics and angiographic characteristics of the two groups were similar. The technical success rate did not differ between magnetic and conventional PCI (95.7 vs 97.8%; p=1.00). Significantly shorter procedural and fluoroscopy time were observed for magnetic compared to conventional PCI (29.9±17.6 vs 41.1±21 min, p=0.007; 7.5±7.3 vs 16.1±22.4 min, p=0.02 respectively). Less contrast was used in the magnetic PCI group (58 ml/patient; P=0.02). These advantages resulted in a mean estimated saving of 1400 euro per patient (P<0.001). Advantages of procedural outcome were even more pronounced in the ACC/AHA lesion class C subgroup.
Conclusions: Magnetic compared to conventional PCI is an attractive novel technique that proved to be feasible and safe and might be faster in distal and especially complex lesions.
Abbreviations
PCI: percutaneous coronary intervention
Magnetic PCI: magnetic guidewire navigation PCI
Conventional PCI: conventional guidewire navigation PCI
MNS: magnetic navigation system
ACC: American College of Cardiology
AHA: American Heart Association
Introduction
Magnetic guidewire navigation in percutaneous coronary intervention (magnetic PCI) is a new technique for steering the front end of an angioplasty wire using a tip-mounted magnet. This technique has been shown to be both feasible and practical.1-5 Magnetic PCI has proved suitable for all lesion types.2-4 Studies in both phantom and humans have suggested potential benefits for magnetic PCI.3,4,6 However, the advantages for different patient subgroups remains undefined.
Magnetic PCI is based on the production of a 2 or 3-dimensional reconstruction of the vessel lumen from standard angiographic images. Knowledge of the positions of the table and image intensifier during angiography allows calculation of the vessel coordinates in real space within the patient’s chest. The applied magnetic field can be changed at any time to redirect the wire tip that may improve navigation through complex and tortuous anatomy. Magnetic PCI could also result in less wire deviation into side-branches.7 Benefits of magnetic PCI are complete omnidirectional guidewire navigation, precise direction of wires using navigational vectors within an adjustable magnetic field and increased accuracy by integrating 3-dimensional imaging modalities and 3-dimensional virtual reconstruction software.7 These characteristics of magnetic PCI could potentially result in reduced procedural and fluoroscopy duration and lower material and contrast consumption especially when it is used in patients with distal and complex lesions.5,8 In addition, lower contrast consumption could possibly result in less contrast induced nephropathy.9 For these reasons, the procedural outcomes of patients having magnetic PCI for distal lesions, with special attention to complex lesions, was compared to concurrent matched control patients undergoing conventional guidewire navigation PCI (conventional PCI).
Methods
Selection of patients
Between January 2007 and May 2007, 375 patients underwent single lesion PCI in the Amsterdam department for interventional cardiology, a large regional tertiary centre. From this patient group, 47 consecutive eligible participants having elective single vessel magnetic PCI for distal and/or complex lesions were included. Distal lesions were defined as all coronary segments except for segment 1, 2, 5, 6 and 11 according to the modified ACC/AHA coronary segment classification. Selected magnetic PCI patients were matched by age, gender and coronary segment with 45 control patients having conventional PCI in the same time period.
Exclusion criteria were simple proximal lesions, multivessel PCI, previous failed conventional PCI for the same target lesion, diagnostic review of another vessel in a staged procedure or inclusion of another diagnostic procedure such as fractional flow reserve measurement or the use of intravascular ultrasound. An ACC/AHA lesion class type C subgroup was defined in both groups. All patients gave informed consent to the proposed procedure.
Magnetic navigation system
The Niobe® Magnetic Navigation System (MNS, Stereotaxis Inc., St Louis, MO, USA), is a fully integrated system (workstation and magnet control equipment) for navigating proprietary catheters and guidewires that have very small magnets at their distal tips. The orientation of these devices is controlled precisely by manipulation of the field generated by two adjustable large neodymium-iron-boron magnets that are mounted on mechanical positioners on either side of the patient. Rotation and translation of these magnets maintain a net magnetic field of 0.08-Tesla within a 20 cm diameter spherical field in the patient’s upper thorax. These permanent magnets are stowed and retracted away from the patient when not in use.
The MNS is fully integrated at our centre with the Integris Allura FD10 cardiovascular imaging system (Philips Medical Systems, Eindhoven, The Netherlands) as shown in Figure 1. The Constellation software included in the Stereotaxis MNS was seldom used for navigation. Most of the cases were performed with either free-style (2D clockwise navigation) or full 3D reconstruction. The operator interfaces with the system via the Navigant™ Navigation Work Station software interface (NWS, Stereotaxis Inc., St. Louis, MO, USA). The NWS has several features that are specifically engineered for interventional cardiology procedures; these features include: 3-dimensional (3D) preoperative navigation that allows the user to import and co-register standard angiographic images and perform “point and click” navigation on the imported image. In addition, the NaviView software (CardiOp-B, Paieon Medical, Rosh Ha’ayin, Israel) allows use of angiographic images from the procedure to generate a 3D vessel reconstruction that can also be used for “point and click” navigation (Figure 2).
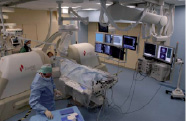
Figure 1. The Niobe® Magnetic Navigation System. A typical magnetic navigation system set-up showing the magnets and C-arm.
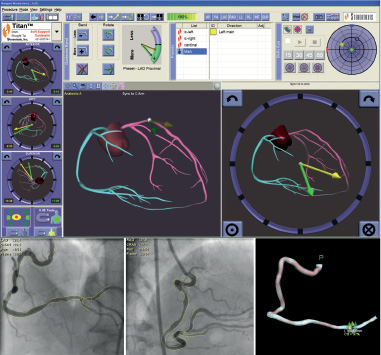
Figure 2. This 3-D reconstruction was created from the fluoroscopic angiogram as shown using the NaviView software feature.
In our centre, choice of wire and navigation technique (2D mode, 3D mode, or a combination of these) was left at the discretion of the operator.
Magnetically enabled wires
A series of magnetically enabled disposable guide wires that are custom designed to integrate with and be precisely navigated by the MNS are available in a variety of sizes and a range of stiffness. For this study, we utilised the Titan™ guidewires that are specifically designed to be more flexible and steerable for magnetic navigation. A schematic of a Titan guidewire is shown in Figure 3.

Figure 3. The Titan® magnetic navigation wire showing the 2 or 3 mm magnet located at its tip.
PCI procedure
The interventional staff of the Onze Lieve Vrouwe Gasthuis includes five experienced operators. Conventional PCI procedures were assigned to all 5 operators whereas magnetic PCI procedures were assigned to 2 of the 5 experienced operators.
Aspergic, 500 mg i.v., was administered before, and clopidogrel, 600mg, was administered after the procedure to all patients. Weight adjusted unfractionated heparin, 100 IU/kg, was given as an intravenous bolus at the beginning of the procedure. The use of glycoprotein IIb/IIIa receptor antagonists was left at the operators` discretion. The approach for access was predominantly the transradial technique or, in case of contraindication, the transfemoral approach. The choice of the guiding catheter and equipment was left at the operators’ discretion and followed routine practice. The angioplasty wires for magnetic PCI were the standard magnetically enabled wires supplied by the company described above. There was no restriction on the operators’ choice of conventional wires. Drug-eluting and bare metal stents were chosen at the operators’ discretion.
Angiographic variables
All angiograms were stored in a computer database and analysed off-line, using CAAS 2003 Camtronics (Phillips Medical System, Eindhoven, The Netherlands), and analysed by an independent observer. Vessel and lesion characteristics have an impact on the performance of the PCI procedure as shown by a study in this issue9.
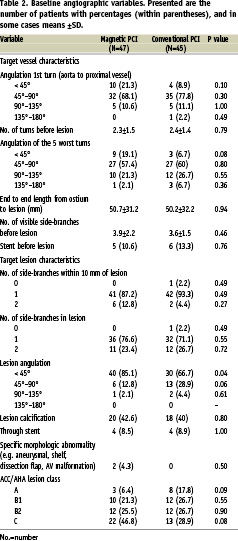
The angulation of the first turn of the vessel is defined as the first angle within the first 30 mm of the vessel in which the catheter is engaged. It is the angle from the ostium at the aorta into the beginning of proximal left anterior descending artery, beginning of proximal circumflex artery, or the first angle into the right coronary artery. The possible grading is <45° (i.e. mild bend), 45°-90° (i.e. moderate bend), 90°-135° (i.e. severe bend) and 135°-180° (i.e. extremely severe bend). The number of turns before the target lesion was noted. The total cumulative angulation of the 5 worst turns was also noted, using the same grading as above. End-to-end length from the ostium to the lesion was measured using quantitative coronary angiography. The number of visible side-branches before the lesion, presence of a stent before a lesion, presence of sidebranches within 10 mm of the lesion, side-branches in the lesion, presence of vessel and lesion calcification were noted. Lesion types were graded according to the ACC/AHA lesion classification.10 Specific morphological abnormalities were also noted (e.g. aneurysm, dissection flap, AV malformation).
Endpoint definitions
Endpoints of this study were to compare procedural outcome in both groups (e.g. technical success, procedural and fluoroscopy duration, contrast and material consumption). Technical success was defined as an intraluminal wire position distal to the stenoses. Procedural success was defined as 1) TIMI grade III flow11, 2) <30% residual stenosis, and 3) absence of clinical events during the procedure (death, acute stent thrombosis, myocardial infarction, emergency coronary artery bypass grafting and stroke). Procedural duration was measured from the time of sheath insertion until the removal of the guiding catheter at the end of the procedure. In case 3D reconstruction was used, procedural duration for magnetic PCI included 3D reconstruction time (±5 min). Fluoroscopy times were automatically recorded by the Poly Diagnost (C2) Digital Cardiac Imaging System (Phillips). Additional endpoints were the occurrence of dissection, stent length and diameter per patient, contrast usage and material consumption. Estimated procedural costs included the materials and laboratory and staff time. Laboratory and staff time were calculated by multiplying the procedural time + 30 minutes by 17 €/minute; the latter figure was based on time-tested cost estimates, obtained from a large data set collected by Cardialysis, a company linked to the university hospital of Rotterdam, the Netherlands. Materials included in the cost analysis were coronary guidewires, angioplasty balloons, premounted bare-metal or drug-eluting stents and angiographic contrast agent.
Statistical analysis
For comparison of continuous variables between the treatment groups, the unpaired two-tailed student’s t test was used or, in the case of skewed data, the Mann-Whitney U-test. Comparison of categorical variables between the 2 groups was performed using the Chi-square test, or in case of a minimum expected count less than 5, the Fisher exact test. Continuous variables were expressed as mean± SD. Categorical data were expressed as numbers of patients and percentage of patients. Spearman rank correlation testing (coefficient Rs) was performed to identify variables related to procedural duration, fluoroscopy time and contrast usage. Among the variables identified, step-down linear regression was performed until all remaining variables were significant, to reveal predictors of procedural duration, fluoroscopy time and contrast usage.12 A P value less than 0.05 was considered statistically significant. Statistical tests were carried out with the SPSS 14.0 statistical software package (Chicago, IL, USA).
Results
Baseline demographics
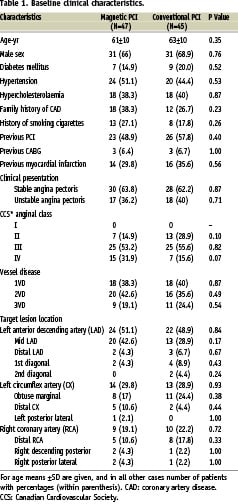
Forty-seven consecutive patients having magnetic PCI for distal and/or complex lesions were compared to 45 matched control patients having conventional PCI. Baseline clinical characteristics (Table 1) did not differ significantly between groups, although angina CCS-class IV tended to occur more frequently in the magnetic PCI group. Most of the baseline angiographic variables (Table 2) were equally distributed between both treatment groups, although the percentage of type C ACC/AHA lesions tended to be higher in the magnetic PCI group (46.6% vs 28.9%; P=0.08). The number of included chronic total occlusions in the magnetic PCI (6.4%) and conventional PCI group (2.2%) were low and did not differ significantly between groups. The occurrence of severe vessel or lesion angulation (90°-180°) was similar between groups.
Procedural outcome
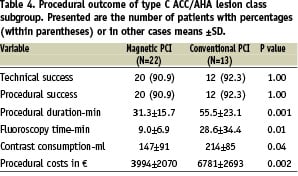
Successful vascular access was obtained via the radial approach in 97% and in the remainder via the femoral artery. Overall technical and procedural success was high (up to 98%) and similar in both groups (Table 3). One serious clinical event occurred in the magnetic PCI group (acute bare-metal stent thrombosis) and one in the conventional PCI group (side branch occlusion). All technical failure cases were type C ACC/AHA lesions. Technical and procedural success was high (up to 92%) in the type C ACC/AHA lesion subgroup (Table 4).
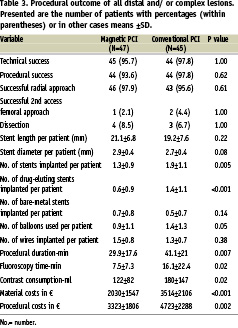
Significantly shorter procedural and fluoroscopy times and lower contrast consumption were observed in favour of magnetic PCI (Table 3). In multivariate analysis, among 12 demographic, clinical and angiographic variables tested, magnetic PCI (P<0.001) and absence of ACC/AHA lesion type C (P=0.01) were independent predictors for shorter procedural duration.
Among 14 demographic, clinical and angiographic variables tested, magnetic PCI (P=0.01), absence of target lesion location in the distal left anterior descending artery (P<0.0001) and absence of 3-vessel disease (P=0.04) were independent predictors of shorter fluoroscopy duration.
Among 14 demographic, clinical and angiographic variables tested, magnetic PCI (P=0.008), absence of a previous myocardial infarction (P=0.006), absence of severe vessel or lesion angulation (<135°; P<0.001) and absence of type C ACC/AHA lesion (P=0.02) were independent predictors for lower contrast consumption.
Advantages in procedural outcome for the magnetic PCI group were even more pronounced in the type C ACC/AHA lesion subgroup (Table 4).
Material consumption and cost estimation
Magnetic PCI was associated on average with an 11 minute reduction in procedural duration (P=0.007) and reduced usage of angiographic contrast agent (58 ml/patient; P=0.02). Significantly more stents and balloons, as well as drug-eluting stents per patient, were implanted in the conventional PCI group (Table 3). Therefore magnetic PCI resulted in significantly lower material and procedural costs compared to conventional PCI, representing a mean procedural saving of 1400 € per patient (P<0.002). Costs remained significantly lower for magnetic PCI when corrected for the difference in number of drug-eluting stents used (2727±1190 vs 3301±1395 €, P=0.04).
The type C ACC/AHA lesion subgroup was larger in the magnetic PCI group (Table 2). In this subgroup magnetic PCI had even more pronounced savings (Table 4) in procedural duration (difference, i.e. delta, with conventional PCI: 24 min), fluoroscopy time (Δ: 20 min), contrast consumption (Δ: 67 ml) and procedural costs (Δ: 2787 €
).
Discussion
Magnetic PCI is a promising technique in cardiac electrophysiological procedures13,14 and is emerging as a powerful tool in interventional cardiology.1-6 Phantom studies have suggested that magnetic PCI provides more benefit in distal and tortuous lesions and may be expected to provide greater advantage in complex procedures.3,4 Therefore, we compared in the clinical setting the efficacy of elective single vessel magnetic PCI in distal and more specifically complex lesions to matched controls using conventional PCI.
Our study indicates that magnetic PCI might be superior to conventional PCI for procedural duration, fluoroscopy time, contrast usage and costs. The high technical and procedural success rates were similar between groups. It is noteworthy that this group of procedures was challenging as is indicated by the time-consuming procedures and high incidence of type C ACC/AHA lesions.
The incidence of type C ACC/AHA lesion, an independent predictor for worse procedural outcome, was significantly higher in the magnetic PCI compared to the conventional PCI group. Therefore the procedural advantage of magnetic PCI could have been more pronounced when ACC/AHA lesions had been equally distributed among groups. It is noteworthy that magnetic PCI was an independent predictor for shorter procedural and fluoroscopy times and lower contrast consumption.
The significantly lower procedural duration, fluoroscopy time, contrast usage, number of stents and balloons used resulted in significantly lower procedural costs with a mean saving of 1400 euro per patient. However, one should bear in mind that the acquisition of the Stereotaxis Magnetic Navigation System is highly expensive (±1.4 million €). At least one thousand patients should be treated with magnetic PCI to compensate for these expenses.
The benefit in procedural outcome for magnetic PCI is even more pronounced in patients having magnetic PCI for type C ACC/AHA lesions (Table 4). In complex lesions magnetic PCI resulted in a 44% reduction of procedural duration, 69% reduction in fluoroscopy duration, 31% reduction in contrast consumption and a 41% reduction in costs. Treatment of these complex lesions is generally more problematic due to the difficulty in steering and pushing a guidewire through them. As the Magnetic Navigation System provides precise, computerised control of the working tip of a guidewire, it can enable physicians to more easily locate small openings in, and to advance a guidewire across, these lesions.
The possible benefit in procedural outcome for magnetic PCI revolves around key abilities of the system. First, magnetic PCI procedures incorporate 3D information (presets, constellation and reconstruction). The automatic 3D alignment of the magnetic tip with the chosen vector, rather than manual steering by estimation from the 2D fluoroscopy screen, improves coaxialisation of the guidewire tip in the vessel. This can help to orientate the guidewire tip in order to pass a vessel angulation, or into a desired branch, and this may reduce incorrect selection of a side-branch, a problem that is inherent with the bent and non-coaxial tip of a conventional wire. This reorientation can be performed within the patient at any time during the procedure meaning that the wire tip is actively steered. This steering is independent of factors in the proximal section of the vessel such as tortuosity or calcium that may impair the rotation and manipulation of a conventional wire that is needed to steer the tip. The result is that the operator is not forced to use a single fixed bend that may not be appropriate to each and every bend of a coronary vessel, lesion and side-branch.
Second, the use of a computer enables the introduction of features that give added value and were previously unavailable during PCI. An example is the white line overlay map on the fluoroscopy screen, which is a further progression as compared to the use of a separate still image on a 2nd screen or ‘roadmap’. The white line overlays the trajectory of the vessel on the fluoroscopy screen, as calculated from the virtual vessel, and is automatically reorientated on the screen as the viewing angle of the x-ray image intensifier is changed. This white line overlay acts as a guide and assists wire placement. This may explain the reduced procedural duration, fluoroscopy time and contrast usage observed in the current study.
In addition to efficient and successful procedures, economic and practical advantages, magnetic PCI results in a substantial reduction in x-ray exposure to physicians, staff and patients as well as potential reduction in contrast induced nephropathy especially in diabetic and hypertensive patients.15,16
This study has limitations associated with cohort studies in that procedures were not randomised. Selection bias could have occurred. As shown in Table 3, stent diameter tended to be larger and the number of stents and balloons implanted per patients were lower for the magnetic PCI group. These findings suggest that relatively larger vessels and shorter lesions were treated in the magnetic PCI group that could in part explain the shorter procedural and fluoroscopy duration and cost advantage in the magnetic PCI group.
Only two out of five operators performed the magnetic PCIs in the current study after a short learning curve, which has been described earlier.6 Operator bias could also have affected outcome. One operator, who performed a large part (±43%) of the conventional PCIs in this study, used significantly more drug-eluting stents per patient compared to the other operators. This could in part explain the higher usage of drug-eluting stents and higher costs in the conventional PCI group. Nevertheless all operators were experienced and multivariate analysis showed that the operator was not a predictor for outcome. In this respect a randomised trial should be performed. However, a randomised trial may also raise objections. It is not possible to blind the performing physician to the type of procedure. All operators should use both techniques in order to eliminate differences in operator skills and speed and the risk of competition between operators with consequences for the quality of the procedure. On the other hand bias could occur in favour of magnetic PCI when all operators perform magnetic as well as conventional PCIs. Another limitation related to magnetic PCI is that certain aspects of the procedure can be time consuming such as obtaining the isocenter position and moving the magnets into the correct position or time to reconstruct. However, with familiarity, these manoeuvres become quick. In addition the choice of magnetic compared to conventional PCI wires is limited especially for flexible wires. This might complicate difficult procedures such as heavily calcified chronic total occlusions. In addition, manipulation of the Titan guidewire in severely angulated lesions could be difficult due to the rigidity of the 2 to 3 mm magnet located at the tip of the guidewire.
In conclusion, magnetic PCI is an attractive novel technique, which proved to be safe and may result in improved procedural outcome in distal and/or complex lesions. A randomised trial is needed for proper comparisons.

