Abstract
Aims: The aim of this study was to evaluate whether there is any relationship between in-stent late loss (ISLL) and the risk of stent thrombosis (ST) in patients treated with drug eluting stents (DES).
The benefit of DES in reducing binary angiographic restenosis and the need for new revascularisation procedures is due to a reduction on ISLL. It has been hypothesised, however, that neointimal hyperplasia could preclude ST, and thus a very low ISLL could increase the risk of ST.
Methods and results: We selected 26 randomised clinical trials comparing bare metal stents and DES or different DES types, and including clinical and angiographic follow-up. In order to evaluate the association between risk of ST and ISLL, meta-regression analyses were conducted, weighting for the number of patients of each study.
Twenty-six studies were included, retrieving 36 subgroups for analysis and 8,971 patients treated with DES. The incidence for ST and LST was 0.81% and 0.17%. Using meta-regression techniques, neither the risk of ST nor the risk of LST were found to be significantly associated with ISLL, accounting for –0.82 and –0.002 meta-regression estimates respectively (IC 95%: –1.92 to 0.28 for ST and –0.008 to 0.003 for LST).
Conclusions: The risk of ST and LST after DES implantation is not related with ISLL values. A very low mean value of ISLL is nor associated with a higher risk of ST.
Abbreviations list
DES: drug-eluting stents
ISLL: in-stent late loss
ST: stent thrombosis
LST: late stent thrombosis
BMS: bare-metal stents
TVR: target vessel revascularisation
Introduction
DES have shown to be more effective than BMS in reducing binary angiographic restenosis and the need of related TVR1-16. This benefit is due to a reduction of neointimal hyperplasia as measured by ISLL. However, concern is growing that DES implantation increases the risk of ST at long-term follow-up, at least after a short time of antiplatelet therapy17-21. It has been hypothesised that incomplete neointimal healing could preclude ST, and thus a very low ISLL could increase the risk of ST22-23. Although uncommon, stent thrombosis remains a severe complication after stent implantation owing to its high morbidity and mortality. Despite reduced restenosis rates, the frequency of in-stent thrombosis has not decreased with DES compared with BMS24-25.
The aim of the present study was to evaluate whether there is any relationship between ISLL and the risk of ST in patients treated with DES. For this purpose, we performed a meta-regression analysis from 26 randomised trials that have evaluated DES and in which routine angiographic follow-up was scheduled.
Methods
Literature review
In order to identify the trials to be included in the study, a systematic review of the literature was carried out on electronic databases (DARE, Cochrane Database, Medline, Embase, Pascal Biomed and Cinahl). The computerised search was done in January 2007 and limited to clinical trials published between 2000 and 2007. The search was performed using the Medical Subjects Heading terms “Angioplasty, Transluminal, Percutaneous, Coronary”, “Stents”, “Drug-eluting”, “Paclitaxel”, “Sirolimus”, “Everolimus”, “Zotarolimus”, “Tacrolimus”, “Thrombosis”. Abstract supplements of major scientific meetings (American College of Cardiology, American Heart Association, European Society of Cardiology and Transcatheter Cardiovascular Therapeutics) were also reviewed. The review was conducted according to the Quality of Reports of Meta-Analysis of Randomised Clinical Trials (QUOROM) recommendations26.
Inclusion criteria
We selected randomised clinical trials that assigned patients to DES versus BMS or DES versus DES and that provided both clinical and angiographic follow-up data, including ISLL and rates of thrombosis, for the patients allocated to DES. As an additional requirement, patients had to be treated with dual antiplatelet therapy, defined as aspirin and clopidogrel, after percutaneous coronary intervention. Studies designed to evaluate DES in acute myocardial infarction were excluded. We included 26 clinical trials that evaluated the following DES: (I) Sirolimus eluting sent (SES) (Cordis Corp, Miami Lakes, FL, USA): RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS, DIABETES, SCANDSTENT, SES-MART, PRISON-II and the study by Pache et al1-9; (II) Paclitaxel eluting stent (PES) (Boston Scientific, Natick, MA, USA): TAXUS-I, TAXUS II, TAXUS IV, TAXUS V and TAXUS VI10-14. As TAXUS II evaluated two different releases of paclitaxel both substudies were separately evaluated. Apart from the trials that compared SES and BMS, we also included the following trials comparing SES and PES: LONG-DES II, ISAR-TEST, REALITY, SIRTAX27,30; (III) Tacrolimus-eluting stent (TES) Janus (Sorin Biomedica, Milan, Italy): JUPITER II trial15. (IV) Zotarolimus eluting stent (ZES) (Medtronic Inc, Minneapolis, MN, USA): ENDEAVOR II, ENDEAVOR III (ZES vs SES), ZOMAXX-I (ZES vs PES)31,33; (V) Everolimus eluting stent (EES) (Abbott): FUTURE I, FUTURE II, SPIRIT II (EES vs PES)16,38. As FUTURE II results were divided into three different groups, we have kept them as separately groups for the analysis; (VI) Biolimus A9 stent (BES) (BioMATRIX™-Stent, Biosensors International, Singapore): STEALTH35.
Definitions
All the studies defined ISLL as the difference between minimum lumen diameter immediately after coronary stent implantation and that obtained at angiographic follow-up. As all trials reported mean values of ISLL, these mean values have been considered in the present study.
ST was defined as an ischaemic clinical event with angiographically proven ST, or clinical events attributable to the target vessel. ST was classified as early, if it occurred within 30 days since stent implantation, or late, if it occurred more than 30 days after stent implantation.
Statistical analysis
Regarding descriptive statistics, quantitative variables are summarised with mean and standard deviation (SD) and qualitative variables with percentages, while inferential statistics are shown with their 95% CI. Correlations between quantitative variables were evaluated with the Spearman rho coefficient.
To evaluate the association between risk of ST and ISLL, as well as risk of late stent thrombosis (LST) and ISLL, meta-regression analyses were conducted, weighting for the number of patients of each study (adjusting models for the inverse variance of the sample size of each study). The estimated β coefficients and their 95% CI were also calculated as well as the R2 coefficient in order to asses the percentage of ST variability explained by each model. To assess heterogeneity among studies a sensitivity analyses was done; all studies that had showed greater changes in the regression coefficient were excluded from the analyses, in order to know whether estimations changed or not. Statistical significance was considered when p value was less than 0.05 (alpha error probability =0.05). Both SPSS 14.0 and CIA statistical packages were used to perform the analysis.
Results
Descriptive characteristics of the included studies
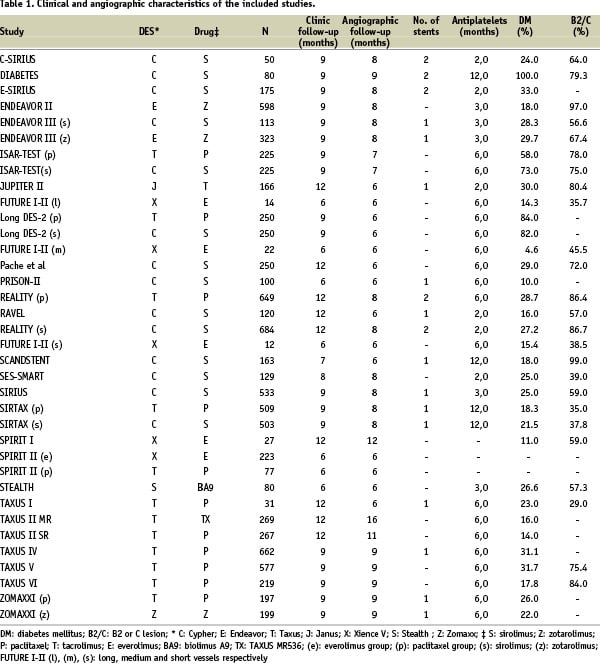
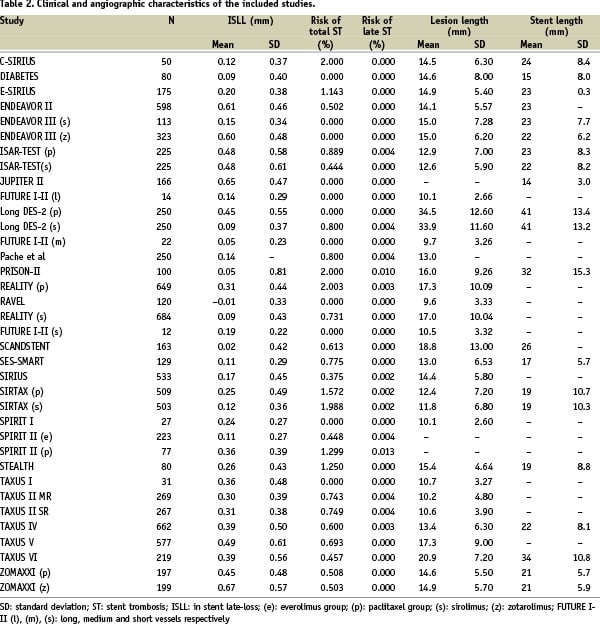
After applying the selection criteria 26 studies were included, retrieving a total of 36 subgroups for analysis and 8,971 patients (range: 12-664). Tables 1 and 2 provide the number of patients included in each subgroup as well as their main clinical and angiographic characteristics.
Inferential estimates of the included studies
Regarding the type of stent, PES was the most frequently used; 43.8% of the patients had PES (95% CI: 42.8-44.9) followed by 37.6% with SES (95% CI: 36.6-38.6). Clinical follow-up of the patients ranged from six to 12 months (mean: 9.08 months; SD: 2.06; 95% CI: 8.38-9.78) and angiographic follow-up ranged from six to 12 months (mean: 7.33; SD: 1.43; 95% CI: 6.85-7.81).
Prevalence of diabetes ranged from 4.6% to 100%, accounting for a total of 2,654 diabetic patients (29.6%; 95% CI: 28.6-30.5). Duration of double antiplatelet treatment ranged from two to 12 months, with a mean duration of 5.55 months (SD: 2.94; 95% CI: 4.50-6.59). With respect to angiographic characteristics, mean lesion length was 14.96 mm (SD: 5.67; 95% CI: 12.94-16.97), and mean stented length was 23.78 mm (SD: 7.37, 95% CI: 20.42-27.13). Mean reference vessel diameter was 2.75 mm (SD: 0.24; 95% CI: 2.67-2.84), and type B2 or C lesions were present in 49.5% of the patients (95% CI: 48.5-50.5).
Relationship between ST and ISLL
The mean value of ISLL was 0.27 mm (SD: 0.19; 95% CI: 0.21-0.34). There were 73 patients with ST (0.81%; 95% CI: 0.62-1.00%) of whom 15 (20.5%) had late ST (0.17%; 95% CI: 0.7-0.26%).
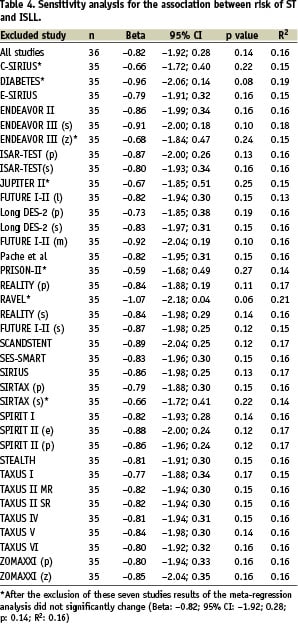
Using the curve fit regression analysis, the best fit was obtained with multiple linear regression (Figure 1), weighting by the inverse variance of the number of patients included in each trial.
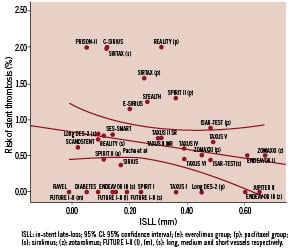
Figure 1. Association between in stent late-loss and risk of stent thrombosis.
No significant association was found between the risk of ST and ISLL (β:-0.82; 95% CI for β coefficient: –1.92 to 0.28; R2: 0.16; p=0.14). Even after adjusting for other relevant variables, that are known to be related to ST occurrence, the association between ST and ISLL remained no significant (Table 3).
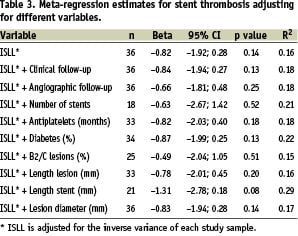
No heterogeneity among studies was detected (Table 4); even after the simultaneous exclusion of all the subgroups that had showed greater changes in the regression coefficient (C-SIRIUS, DIABETES, ENDEAVOR III-zotarolimus group, JUPITER II, PRISON II, RAVEL and SIRTAX-sirolimus group), final estimates did not change (β: –0.82; 95% CI: –2.91-0.28; R2: 0.16; p=0.14).
Relationship between late ST and ISLL
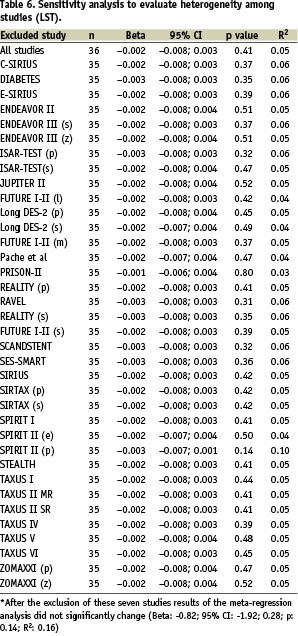
Figure 2 and Table 5 show the lack of association found between LST and ISLL, even after adjusting for relevant variables (β: –0.002; 95% CI for β coefficient: –0.008 to 0.003; R2: 0.05; p= 0.41).

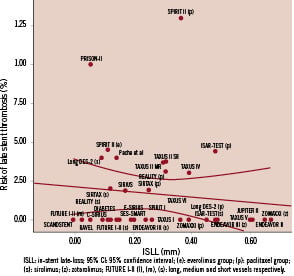
Figure 2. Association between in stent late-loss and risk of late stent thrombosis.
The sensitivity analysis for late ST retrieved no differences among studies (Table 6).
Discussion
Risk of ST
In this analysis, including 26 studies in 8,971 patients treated with DES, the incidence of ST after DES implantation was 0.81%, observed in a mean follow-up of over nine months. This overall rate is comparable to that observed in previous studies with DES19 and similar to that reported for BMS40. The incidence of LST, evaluated more than 30 days after the procedure, was 0.17%. The meta-analysis of Bavry et al reported a lower risk of late ST, accounting for five events per 1,000 DES patients17; our higher incidence might be due to the additional studies that have been included in the analysis, which reported greater risk of LST. Some registries, in which selection of the patients was not controlled, have shown higher LST incidence, Duk-Woo et al37 reported a 0.6% risk as well as BASKET-LATE18 study found a risk of 1.4%, probably due to the fact that they included more complex lesions, with longer follow-up (median time of follow-up of 15-18 months) and associated to clinical situations different from stable angina. However, because clinical follow-up of most of the published pooled trials is limited up to one year, at the moment, the risk for very late ST (ST occurring more than one year after the procedure) remains unclear. Therefore, the risk of LST in the “real clinical world” might be considerably higher than that retrieved for clinical trials, and even higher if misdiagnosis of those angiographically not documented LST cases were taken into account.
All patients included in the present study had to be under double antiplatelet therapy (aspirin and thienopyridines) for at least two months and had a medium duration of treatment of 5.5 months. The premature thienopyridine cessation has been reported as the most striking factor associated with ST with a risk ranging from 14.8 to 30.0%38,39. The appropriate duration of the long-term antiplatelet regimen for prevention of DES thrombosis remains to be assessed in randomised prospective trials, but the most recent consensus recommends administering double anti-platelet therapy for one year after DES implantation40.
ISLL and risk of ST
ISLL reflects the biological effect of DES in a very precise way since lower ISLL levels correspond to less neointimal formation. Some studies have shown that ISLL is a valid parameter to evaluate the efficacy of DES41; moreover, we have recently demonstrated that ISLL is associated with the clinical benefit derived from the use of DES, since as ISLL decreases, the number of patients needed to be treated to prevent one revascularisation procedure also decreases42.
However, whether ISLL is related with the safety of DES has not been clarified. It has been hypothesised that the reduction of the neointimal hyperplasia produced by DES, might preclude a failure to form a complete neointimal layer over the stent struts so that it would extend the time during DES would be prone to thrombosis as shown with coronary brachytherapy43. Experimental studies and analysis of post-mortem human coronary artery stents have shown a pathological response to the arterial injury after stenting. Neointimal healing, containing smooth muscle cells in a collagen and proteoglycan extracellular matrix covering stent struts, has been identified in BMS in the first month44.
DES with LST have showed more delayed healing compared with patent DES in a registry of 40 autopsies45. In animals treated with rapamycin, delayed neointimal healing has been observed, resulting in persistent fibrin deposition46. The endothelium plays an important role in preventing thrombus deposition since it seals the thrombogenic components in the underlying artery from the blood, providing protection against ST. It is still unclear whether there is any relationship between neointimal healing and endothelisation, how they are affected by the different drugs delivered, and which plays the principal role in thrombosis prevention.
DES with lower values of ISSL have not showed a higher risk of ST as compared to those with worse angiographic results1-16. The risk of ST after SES implantation is not higher than that after PES implantation, despite the former having lower ISLL values47. Many clinical and angiographic factors have been described to be associated with a higher risk of ST: stent length19, late stent malapposition11, aneurysm formation, primary stenting in acute myocardial infarction37, localised hypersensitivity to the polymer, by-pass grafting, side-branch occlusion, need of IIb/IIIa inhibitors18, renal failure, bifurcation lesions, diabetes, and a lower ejection fraction21. None of these factors has been shown to be related with an impaired arterial healing.
In the present study, neither the risk of total ST nor the risk of LST were found to be significantly associated with ISLL, accounting for –0.82 and –0.002 meta-regression estimates respectively (IC 95%: –1.92 to 0.28 for ST and –0.008 to 0.003 for LST). These results might reflect the lack of association between the neointimal hyperplasia inhibition, which is the corner stone of the clinical benefit of DES over BMS, and the risk of thrombosis.
In conclusion, neither statistical nor clinical significant relationship between the risk of DES thrombosis and ISLL was found in the present study, especially regarding LST, not even after adjusting for different relevant variables that are supposed to increase the risk of ST. These results may be partly due to the low incidence of ST and also to the lack of statistical power of the present study, which works with aggregated data, and where only 16% of the total ST risk and 5% of the late ST risk could be explained by ISLL. Furthermore, the pathogenesis of stent thrombosis is still not understood. A combination of factors may be involved, including periprocedural related factors, patient-related factors, and lesion characteristics.
Study limitations
First, due to the meta-regression technique used, this study might have missed some confounder variables, since it is an inherent limitation of these specific analyses. Second, ISLL may vary with some patient and lesion characteristics, such as diabetes or lesion length, however, both regression models had been previously adjusted for these variables and results had not changed. Another related and unavoidable limitation is that ISLL may also vary among different stent platforms and laboratories. Forth, heterogeneity among studies may produce distorted results, so that, as trials did not have similar sample sizes and, in order to avoid bias, meta-regression models were adjusted for the inverse variance of the sample size of each study. Additionally, a sensitivity analyses was done and no heterogeneity among studies was detected, even after the simultaneous exclusion of all the subgroups that had showed greater changes in the regression coefficient. Recent pooled analysis have shown a slight increase (0.1-0.2% per year) in the risk of ST more than one year after stent implantation with DES. Most of the trials included in this study have a relatively short follow-up, which may lead to an underestimation of the real risk of LST.
Finally, recent studies48 have argued that late loss is not a reliable marker of the true efficacy of DES devices due to its complex and non-Gaussian distribution. However, it seems to be a controversial issue to study in depth in future studies.

