With the publication in this issue of the study by Rivero et al,1 appraising the association between stent thrombosis and late lumen loss, the clinical impact of the latter is again challenged. Yet, before witnessing the possibly last (scientific) breaths of late loss, it is appropriate to challenge our definition and operational knowledge of this important, but often misused and misinterpreted angiographic parameter.
What is late lumen loss?
Most interventional cardiologists have a rather clear idea of this angiographic parameter obtained at quantitative coronary angiography by subtracting the follow-up minimum lumen diameter to the post-procedural minimum lumen diameter (Figure 1A). Given that with currently available stents recoil is minimal and thus in-stent constrictive remodelling unlikely, late lumen loss appears as a good estimator of in-stent neointimal hyperplasia. As such it has been used in several clinical studies as a surrogate endpoint for clinical efficacy of coronary devices.2
How does late loss behave?
Despite our common assumption that most continuous biological variables have a Gaussian (i.e., normal) distribution, the exact behaviour of late loss in large samples of patients remains debated.3 Indeed, most early studies presented and analysed late loss assuming an underlying Gaussian distribution or, in the worst case scenario, a unimodal right skewed distribution (Figure 1B).4 However, a number of investigators have definitely shown that a bimodal distribution appears to better suit the distribution of late loss.2-3,5-6
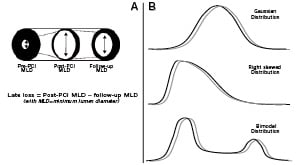
Figure 1. Panel A: Scheme of an artery before, just after and long after percutaneous coronary intervention (PCI), with the definition of angiographic late lumen loss, as post-PCI minimum lumen diameter (MLD) minus follow-up MLD. Panel B: Examples of frequency distributions of continuous biological variables. Despite being commonly reported as following a Gaussian distribution (top), late loss has been presented in some cases as distributed in a right-skewed fashion (middle), but the most appropriate distribution summarising late loss values in real patients remains a bimodal distribution (bottom). Conversely, patient age typically follows a Gaussian distribution (top) in clinical studies, and post-procedural peak cardiac enzyme levels most commonly follow a right-skewed distribution (middle).
What is the clinical impact of late loss?
According to an obvious logic associating low late loss with low neointimal hyperplasia and thereby low restenosis, in the quest for the most effective coronary device, the interventional community has enthusiastically sought those devices associated with the lowest late loss. Eventually this approach has lead to the identification of drug-eluting stents (especially sirolimus and everolimus eluting stents) as the most efficacious and apparently safe devices.7-8
The impact of late loss appears all too evident in routine clinical practice, especially in medium (e.g. with reference vessel diameter < 3.0 mm) or small diameter (< 2.5 mm) coronary vessels, where a low late loss should correspond to lower values of diameter restenosis at follow-up, and reduced rates of binary angiographic restenosis. On the other hand, the impact of late loss in larger vessels (e.g., with reference vessel diameter > 3.5 mm) remains unclear, unless frank restenosis occurs.
Proof of such a concept has been reiterated in many trials during the last two decades, and has been also recently summarised in an extensive systematic review by Moreno et al.9 In this work, the authors performed a meta-regression analysis testing across 21 studies and 8,641 patients the impact of late loss on the risk of target lesion revascularisation. Meta-regression is a specific type of systematic review exploiting meta-analytic pooling techniques which enable the appraisal of associations between a specific dependent variable (in this case target lesion revascularisation) and one or more independent variables of interest (in this case late loss). The subtlety of meta-regression is that each study is pooled with the others while not dismissing its size and statistical weight, thus large studies have a much larger impact than smaller studies (or those with few events despite a large sample). In their work, Moreno et al demonstrated a significant association between average per-study late loss and per-study rates of target lesion revascularisation (p<0.01). Thus, despite the fact that “average” late loss is a misnomer given its established bimodal distribution, using such “average” values of late loss may provide relatively robust guidance in estimating the risk of repeat revascularisation with a given device.
Beyond the plausible association between clinical restenosis and late loss, Camenzind et al have purported that late loss could be a proxy for healing processes, and thus that very low (and especially negative) late loss values could identify cases with incomplete or absent healing (i.e., lack of endothelialisation and strut coverage) leading to late and very late stent thrombosis.10 Such view has been embraced by several critics of drug-eluting stents amid concerns that coronary devices associated with very low late loss values (e.g., sirolimus and everolimus-eluting stents) could lead to higher rates of stent thrombosis in comparison to drug-eluting stents associated with relatively higher late loss values (e.g., zotarolimus-eluting stents with phosphoryl choline coating) or in comparison to bare-metal stents (typically associated with much higher late loss).
Rivero et al1 report in this issue on a meta-regression analysis focusing on the association between late loss and stent thrombosis. A total of 26 studies with 8,971 patients treated with either drug-eluting or bare-metal stents were retrieved, and meta-regression analyses weighting each study according to its sample size showed that the incidences of stent thrombosis and late stent thrombosis were 0.81% and 0.17%, respectively. Moreover, neither the risk of stent thrombosis, nor the risk of late thrombosis, appeared significantly associated with in-stent late loss (p=0.14 and p=0.41, respectively). Given these findings, an association between stent thrombosis and late loss is to date unlikely, and other phenomena and mechanisms, beyond neointimal hyperplasia, likely interact in the pathophysiologic cascade leading to stent thrombosis. Indeed, those viewing extremely low late loss values as evidence of incomplete healing clearly leading to thrombosis now appear frankly naive.
What should we do with late loss in the future?
Angiographic late lumen loss remains a useful angiographic surrogate endpoint in clinical studies of coronary devices, but its surrogacy should always be borne mind. This appears all to evident, given its bimodal distribution,2 the variability in the association between late loss and repeat revascularisation,9 and the lack of association between late loss and stent thrombosis.1 Similar arguments apply to other angiographic parameters, which have been proposed as optimal surrogates but which may risk misleading researchers, clinicians, regulatory authorities, and patients.11
Thus, we can continue to exploit the mechanistic and pathophysiologic relevance of late loss and other angiographic parameters, yet we should remain concerned about their inherent limitations and lack of clear clinical impact. Only large randomised clinical studies, adequately powered for clinically-relevant endpoints, will remain able to provide meaningful answers for regulatory approval or routine clinical use of new-generation coronary devices.
Acknowledgements
This work is part of a senior investigator research program of the Meta-analysis and Evidence-based medicine Training in Cardiology (METCARDIO) Center, based in Turin, Italy (http://www.metcardio.org).

