Introduction
This keynote lecture will address the following objectives: 1) to describe the natural history of patients with severe aortic stenosis who, for a host of reasons, are denied conventional surgical aortic valve replacement; 2) to review the clinical predictors of mortality and morbidity following surgical aortic valve replacement (AVR); 3) to describe the outcomes of surgical aortic valve replacement in high risk patients i.e. octogenarians and patients with left ventricular dysfunction; 4) to highlight the “unmet clinical need” for surgical aortic valve replacement in Europe; 5) to describe the potential advantages of transcatheter aortic valve replacement therapies (TAVR); 6) to describe the haemodynamic and clinical outcomes of the CoreValve ReValving® System and the Edwards Lifesciences SAPIEN™ prosthetic heart valve for TAVR; 7) to review the global market forecast of heart valve therapies; and finally, 8) to provide a “glimpse into the future” of newer generation transcatheter aortic valve devices.
Basic principle
There is no question that, in spite of the compelling reasons so eloquently exposed in the surgical literature, patients and treating physicians will always prefer a lesser invasive procedure as long as there is an equivalent safety and efficacy. Furthermore, a lesser invasive treatment offers the promise of a better option than medical management for those patients who are truly inoperable. Hypothetically speaking, if it can be demonstrated that transcatheter valve implantation or repair can be done as safely as surgery, and with an equivalent short- and long-term efficacy, it may expand or even supplant some current surgical treatment options. This is a very proactive, but sound, hypothesis and it does not intend to detract in any way from the skills of the cardiac surgeons or the excellent results that have been achieved with conventional aortic valve replacement over the past 40 years.
Natural history of nonsurgically managed patient with severe AS
We selected the largest and longest follow-up study that examined the natural history of non-surgically managed patients with aortic stenosis1. In this study, 740 patients with severe aortic stenosis (aortic valve area ≤0.8 cm2) were identified by echocardiography. Of these patients, 453 patients (61%) did not undergo surgical aortic valve replacement and formed the cohort of the study. The exact reasons for not undergoing surgery predominantly included: (i) lack of symptoms; (ii) patient refusal to undergo surgery; and (iii) too high surgical risk candidates. The mean age of the patients was 75±13 years and in one-third of patients the left ventricular ejection fraction was ≤40%. In these non-surgically managed patients, the mortality at 1-, 5-, and at 10-years was 38%, 68%, and 82%, respectively. Thus, it is reasonable to assume that the prognosis in the medically managed but untreated patient has changed little from that offered from Ross and Braunwald over 40 years ago2 (Figure 1).
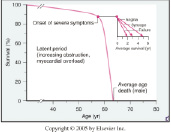
Figure 1. The onset of symptoms in patients with aortic stenosis heralds a poor prognosis (From Braunwald E, Zipes D, Libby P, Bonow, R. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed: Saunders, 2004). With kind permission from Elsevier.
Is there a benefit to surgical aortic valve replacement in elderly patients?
Varadarajan and colleagues examined the survival of octogenarians in need of AVR3. The cohort was comprised of 277 patients with severe aortic stenosis and ≥80 years of age. Of these, 55 patients (20%) underwent AVR. The study demonstrated a significant survival benefit for patients undergoing AVR, even after propensity score analysis (87 vs. 52% at 1-year, 78 vs. 40% at 2-years, and 68 vs. 22%, p < 0.001) (Figure 2).
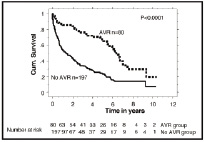
Figure 2. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: Results from a cohort of 277 patients aged ≥80 years (From Varadarajan P, Kapoor N, Bansal RC, Pai RG.Eur J Cardiothorac Surg 2006;30:722-7). With kind permission from Elsevier.
We concur with the late cardiac surgeon Dwight Harken, MD (1910-1993) who performed the first successful aortic heart valve replacement, and who said “a device is safe when the risk of not using it is greater than the risk of using it.” This may very well be the case again today for our inoperable elderly patients with severe symptomatic aortic stenosis who can benefit from the advent of transcatheter aortic valve technologies.
Outcome of surgical aortic valve replacement
An initial review of the results of the US Medicare database from 1994 to 1999 comprised of 143,408 AVR procedures performed in 684 hospitals showed an overall in-hospital mortality of 8.8%. The in-hospital mortality rate in high and low volume centres was 6.0% and 13.0%, respectively4. These data are, of course, one decade old and more recent studies indicate an overall in-hospital mortality rate of 5.6%5.
Predictors of mortality and morbidity after surgical aortic valve replacement
During the same decade, authors from eight northern New England medical centres identified predictors of mortality in 5,793 patients who underwent AVR6. In the multivariate analysis, 11 independent variables were identified. The 5 major predictors of mortality were: 1) age ≥80 years; 2) New York Heart Association class III and IV; 3) left ventricular ejection fraction <30% associated with prior myocardial infarction; 4) emergent aortic valve replacement; and 5) concomitant coronary artery bypass graft surgery. Other minor predictors included female gender, body surface area, elevated creatinine concentration, atrial fibrillation, prior cerebrovascular disease, and prolonged cardiopulmonary bypass time.
With these variables in mind, experimental and clinical studies have shown that cardiopulmonary bypass and cardioplegic arrest can have adverse consequences. More specifically, systolic and diastolic dysfunction can be the result of: i) non-pulsatile flow, low perfusion pressure7,8; ii) embolic events caused by gas or blood-borne products9; iii) myocardial oedema10; and, iv) systemic inflammatory response syndrome10. Cardiac surgery can also be associated with post-operative organ dysfunction. Some degree of myocardial injury following cardiac surgery is almost universal. Nesher and colleagues reported elevated troponin levels above the upper limit of normal in 90% of patients following cardiac surgery11. Furthermore, 40% of patients had troponin elevations greater than eight times the upper limit of normal. Elevations in troponin levels were independently associated with increased major adverse cardiac events. Acute renal failure can be observed in up to one third of patients after cardiac surgery and renal replacement therapy can be required in 1-5% of patients. The peri-operative mortality associated with post-operative renal failure has been reported to range between 28-63%12-14. Blood transfusion, required in up to one third of patients, has been associated with a 2-fold increase in 5-year mortality15,16.
Table 1 summarises a review of more recent data.
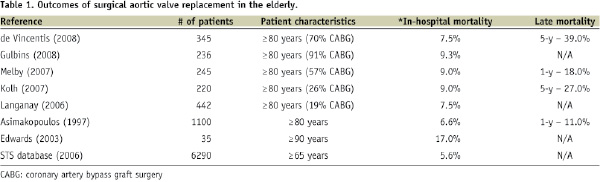
Rankin et al analysed the results of 409,100 valve procedures from the Society of Thoracic Surgeons (STS) database (1994-2003)5. Of the 216,245 patients who underwent isolated aortic valve replacement, the unadjusted and adjusted mortality rates were 5.7% and 5.6%, respectively. It must be noted, however, that data entered into the STS database is voluntary (i.e. reporting bias) and is estimated to capture approximately 70% of the cardiac surgical procedures performed in high and low volume centres across the United States.
In the largest reported series of 1,100 octogenarian patients undergoing AVR, the 30-day and 1-year mortality rates were 6.6% and 11%, respectively17. In recent medium-sized cohort studies of octogenarians undergoing AVR, the in-hospital mortality rate has ranged from 4.6-13.5%17-24 (Table 1). In another study of 220 octogenarian patients, the rate of in-hospital mortality was 13% overall, 9% for aortic valve replacement alone, 24% for combined aortic valve replacement and coronary artery bypass surgery, and 32% for urgent procedures22. This study highlights the influence of the patient’s pre-operative clinical status on survival following AVR.
We have just reviewed the outcome of AVR as a function of age. What is the outcome of patients with left ventricular dysfunction and aortic stenosis (i.e. low flow/low gradient aortic stenosis) undergoing this surgery? Over the last 3-years it has become apparent that the in-hospital mortality rate can range from 6% to 33%, depending to a large extent on the presence or absence of contractile reserve25. This was further corroborated by Subramanian et al who published 14 best evidence papers on this topic and concluded that patients with low flow/low gradient aortic stenosis (i.e. left ventricular ejection fraction ≤40% and mean transaortic valve gradient < 30 mmHg) and no contractile reserve have a surgical mortality risk as high as 30%26.
Unmet need
In an attempt to define the prevalence of aortic valve stenosis by severity and morbidity, a chart has been created to reflect the indications for aortic valve replacement (Figure 3).
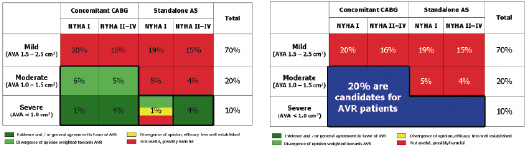
Figure 3. Chart describes indications for surgical aortic valve replacement in patients with or without the need for coronary artery bypass surgery. Approximately 20% of patients with aortic stenosis (highlighted in blue) have an indication for aortic valve replacement.
To establish this chart we consulted the ACC/AHA heart valve guidelines27, the Society of Thoracic Surgeons Database (2003), and the EuroHeart Survey Study28. In patients with isolated mild-moderate aortic stenosis, and in patients with mild aortic stenosis in need of coronary artery bypass surgery, AVR is not considered useful and may be possibly harmful. Although there is a divergence of opinion, patients with moderate aortic stenosis in need of coronary artery bypass surgery are advised to undergo aortic valve replacement during the same procedure. There is consensus that aortic valve replacement should be performed in patients with severe aortic stenosis who are symptomatic and/or have left ventricular dysfunction and/or are in need of coronary artery bypass surgery. The optimal treatment of patients with severe aortic stenosis who are asymptomatic or have normal left ventricular function is unclear. From Figure 3 it can be noted that approximately one-fifth patients with aortic stenosis have an indication for aortic valve replacement.
When we try to define the prevalence of the need for aortic valve treatment, albeit surgical or transcatheter, we should realise that in the European Union 77 million people are older than 65 years. If we assume a prevalence rate of aortic stenosis of 4%, then the numeric prevalence of the disease in Europe is 3 million28. If 20% of patients with aortic stenosis have an indication for aortic valve replacement, then there are 600,000 patients in need of aortic valve replacement. If one considers that only 60,000 aortic valve replacements are performed annually in Europe, we can surmise that an “unmet clinical need” clearly exists.
These theoretical analyses fully justify the statement made by one of my best surgical friends, Fredric Mohr, co-principal investigator of the SYNTAX Trial, when he noted, “For some time we have believed that there is a substantial population of aortic valve disease patients who, today, are under treated. Early demographic data suggest that for every patient who receives surgery, there is another who is severe and symptomatic enough to benefit from a replacement, but does not receive one”.
Potential drivers of the transcatheter technologies
As discussed previously, the demographics are favourable for transcatheter aortic valve implantation. It is still unclear, however, if TAVR may obviate the need or reduce the number of surgeries in a patient’s lifetime. The new approach is percutaneous transfemoral, subclavian, or transapical and thus less invasive than traditional open heart surgery. It eliminates the need for cardiopulmonary bypass and cardioplegic arrest, and some TAVR technologies even allow for elimination of full anaesthesia in selected patients. As a result, the hypothesis is that patients will face less complications (e.g. infection, transfusion), and, less emotional and physical pain. In addition, TAVR will allow for a shorter recovery period and less discharge to rehabilitation centres. From a cosmetic point of view, a large scar on the chest will be avoided. As usual, the cost-effectiveness will be a subject to debate and will be resolved only once the “true” financial value of the devices are established. A new alliance between the cardiologist and cardiac surgeon has emerged out of the necessity to establish a program with a triple facet of treatment, i.e. conventional surgery, transfemoral and transapical aortic valve implantation; and this has become one of the unexpected and paradoxical drivers of this new technology. Institutions capable of treating their patients through a multi-disciplinary approach that includes all these diverse approaches will benefit from a better referral of patients with aortic valve stenosis – a phenomenon we also observed in the past with coronary artery bypass graft surgery and percutaneous coronary intervention.
Perhaps ironically, the most prolific team of doctors currently performing transfemoral implantation of the CoreValve ReValving System in Europe are the surgical team from the German Heart Centre Munich (Drs. R. Lange, S. Bleiziffer, and D. Mazzitelli). Since starting their transcatheter program in mid-2007, the team has been certified on both the CoreValve and Edwards Lifesciences technologies and has performed almost 150 total cases to date. Approximately 15-20 cases are now performed each month and an additional 200 cases are forecast for the year 2008.
Outcome data
On April 15, 2007 and Aug 31, 2007 the CoreValve ReValving System and the Edwards Lifesciences SAPIEN prosthetic heart valve obtained CE mark approval respectively. In the last 5 years, over 4000 transcatheter aortic valve implantations have been performed worldwide using these two differing technologies, with about 85% of these procedures taking place in the year since the European market clearance. There is a compelling sense of familiarity or eeriness (i.e. déjà vu) in this last statement considering that in 1980, 3000 percutaneous transluminal coronary angioplasties had been performed worldwide since being first introduced by the late Dr Andreas R. Gruntzig (1939-1985) in 1977.
The CoreValve ReValving System is a self-expanding bioprosthetic heart valve. It has a multi-level frame composed of nitinol to which is sewn a trifoliate porcine pericardial heart valve. Two sizes are currently available for clinical use – 26 mm and 29 mm inflow size devices for treatment of patients with an annulus between 20 and 27 mm29.
The Edwards Lifesciences SAPIEN device is a balloon-expandable bioprosthetic heart valve. In this case, a trifoliate bovine pericardial heart valve is sewn to a stainless steel “stent-like” structure. Two sizes are currently available for clinical use – a 23 mm and 26 mm device for treatment of patients with an annulus between 18 and 24 mm30.
The procedural success with the two devices has reached a high value above 95%. The procedural success in the recent 18 Fr CoreValve registry study and in the Vancouver SAPIEN experience (i.e. last 75 patients using the newest generation Edwards Lifesciences RetroFlex catheter delivery system) was 97% and 100%, respectively. A significant decrease in transaortic valve gradient can be achieved with both devices (Figure 4).
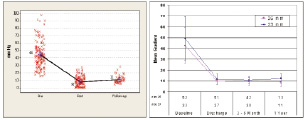
Figure 4. Following transcatheter aortic valve implantation there is a significant decrease in the transaortic valve gradient. A) CoreValve ReValving system (21 Fr and 18 Fr Safety and Efficacy Study, n=175). B) Edwards Lifesciences SAPIEN prosthetic heart valve (REVIVE II and REVIVAL II and Canadian Special Access, n=193).
Of note, grade 1 or 2 paravalvular aortic regurgitation can be observed in 60-70% of patients. Interestingly, paravalvular leakage can also be identified in 6-48% of patients after AVR31,32. While much discussion has (and will) take place regarding this challenge, we recall the statement by cardiac surgeon, Dr. M. Ionescu that, “small paraprosthetic leaks are common, are related to surgical factors, are not associated with increased subclinical haemolysis, and are benign during the first year after heart valve replacement.”
Of note, the 30-day mortality rate in the CoreValve registry of 646 patients was 8.1%, a number very similar to the Vancouver experience (n=112) of 8%. Taking into consideration the age, comorbidities and the high or prohibitive surgical risk (or documented inoperability) of the patients, a 30-day mortality rate of 8% compares quite favourably to the results of high-risk cohorts receiving surgical aortic valve replacement.
Undoubtedly, neurological events and myocardial infarction are still reasons for concern (Table 2).
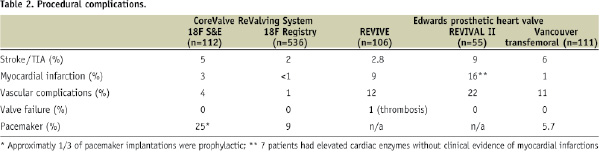
It would seem reasonable to believe that vascular complications would occur less frequently with the CoreValve 18 Fr vascular access sheath than the Edwards Lifesciences 22 Fr or 24 Fr vascular access sheath. The procedure can induce conduction defects and permanent pacemaking can also be required. At this time, however, the indications for permanent pacemaking following transcatheter aortic valve implantation are unclear. After surgical aortic valve replacement, approximately 6.0-6.5% of elderly patients require permanent pacemaking. Further observation and research are needed to clarify the “true” indications for permanent pacemaking. It is reassuring to see that valve failure is non-existent at medium term follow-up. There is an improvement of functional status overtime (Figure 5).
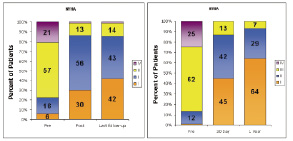
Figure 5. Following transcatheter aortic valve implantation there is significant improvement in functional status. (A) CoreValve ReValving system (B) Edwards Lifesciences SAPIEN prosthetic heart valve. It is noteworthy that greater than 80% of patients were in NYHA class I or II at 1-year or last follow-up; in comparison, greater than 75% of patients were in NYHA class III or IV before treatment. At 6-months, the survival rate is remarkably similar with both devices (Figure 6).
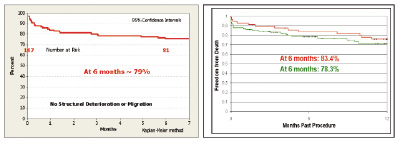
Figure 6. At 6 months follow-up, the survival rate is approximately 80%. (A) CoreValve ReValving System (18F registry, n=107). (B) Edwards Lifesciences SAPIEN prosthetic heart valve (REVIVE II n=106 (green) and REVIVAL II n= 55 (red)).
The goal of this keynote address was NOT to contrast the results of the two technologies, but to highlight that both technologies have contributed in a similar fashion to the exploration of a new frontier in the treatment of structural heart disease and ultimately to the benefit of elderly patients with substantive AVD.
Market forecast
It is unusual for me to rely on the market assessments made by company investors or analysts – it does not belong to my intellectual or scientific beliefs. It is true, however, that these market assessors have become, over the years, experts in predicting the prognosis or success of medical devices and/or drugs in cardiovascular medicine. Therefore, we have to pay particular attention or at least consider seriously, their contention in market development.
The market value of mechanical and tissue valves, as forecast by the Millenium Research Group (MRG), will not change substantially over the next 5 years34. In contrast, an exponential rise in market value of transcatheter heart valve therapies is expected. In this regard, MRG predicts percutaneous heart valve therapies will posses a market value of approximately 700 million US dollars by 2012. Market assessments by similar organisations see these numbers as conservative.
MRG estimates the ratio of the number of transcatheter heart valve procedures to total heart valve procedures will increase from <1% to 40% from 2007 to 2012, respectively. I would have considered these assessments as preposterous, were it not for the total number of procedures that have been performed since CE mark approval. The Edwards Lifesciences SAPIEN® and CoreValve ReValving Systems are currently established in more than 20 countries, performed in more than 80 sites each and we estimate that at least 250 TAVI cases are performed each month worldwide. Remarkably, just 1-year ago less than 10 countries involving less than 10 sites were performing less than 10 cases per month worldwide.
This evolution raises many questions about the future of the CE mark of such devices. In Europe, three types of medical practices exist. Firstly, there are centres with percutaneous coronary intervention programs and cardiac surgery on-site that work in close collaboration and discuss the optimal treatment strategy of patients referred for coronary and/or valve disease. We can reasonably expect these centres to respect and adhere to the so-called CE mark labelling indications. Secondly, there are institutions with percutaneous coronary intervention and surgery on-site, but who do not do not partake in inter-disciplinary discussion regarding the treatment of patients referred for coronary or valve disease. In essence, these institutions have two distinct referral and practice patterns. In this situation, progressive broadening for the indications of percutaneous interventions can be expected. The third type of practice includes percutaneous coronary interventions without surgery on-site. A recent survey in the European Union concluded that the percentage of “isolated” PCI centres is 37%.35 Today, we know the companies can and will restrict access to the devices in accordance with labelling, however, we all know their capacity for self-restraint will have commercial limits.
With increasing operator experience and access to the device, physicians may also become tempted toward a process of self-referral of younger patients with less co-morbidities, the current so-called “surgical candidates”. Although this practice of unrestrained referral can be viewed as hazardous, it might provide the incentive for conducting randomised controlled trials in patients with less surgical risk. Similar observations have been made in the past regarding surgical and percutaneous coronary revascularisation. Once non-evidence-based medicine is performed, evidence-based medicine will need to follow practice-based medicine. Percutaneous treatment of left-main disease and/or 3-vessel disease was being performed routinely prior to the SYNTAX trial despite the lack of evidence from randomised controlled trials. With the results of the SYNTAX trial now available36, some myths about percutaneous treatment of left main disease have been mitigated.
Randomised clinical trials and transcatheter aortic valve implantation
While in Europe it might take some time before the design and completion of a randomised controlled trial, Edwards Lifesciences has already initiated a pivotal trial in the US, within the framework of the USA FDA regulatory process towards market clearance. More than 250 patients have been randomised to date in the Edwards Lifesciences PARTNER IDE Trial. The trial will be performed in a double randomised manner following an operative assessment of the patient. If patients are deemed operable, then depending on the quality of femoral access, patients will be randomised to one of two subgroups. If femoral access is possible, patients will be randomised to transfemoral or conventional aortic valve replacement. If transfemoral access is not feasible, patients will be randomised to transapical or conventional aortic valve replacement. Patients deemed inoperable but in whom femoral access is possible, will be randomised to medical or transfemoral aortic valve implantation. Patients who are considered inoperable but have unacceptable femoral access will not be included in study.
This study aims to provide answers to four fundamental questions raised by these disruptive technologies. Firstly, does the technology work? Secondly, if so, work for whom? Thirdly, at what cost? Fourthly, how does it compare with the gold standard? Importantly, quality of life data will also be collected and reported. These questions relate to the quality, safety, and efficacy of the treatment in question. Following these major steps, we will have to face the fourth and final hurdle of basic medical evidence i.e. the cost-effectiveness and related reimbursement. The cost-effectiveness will depend on the price tag of the device. The manufacturer will argue that they will need to be reimbursed for the capital investment made during the research and development. History will tell us that the initial calculations of cost-effectiveness will be unfavourable since the cost of the device is frequently excessive. The cost-effectiveness relationship becomes favourable once competitive companies enter the market and introduce newer devices at lower prices.
Shortly after CoreValve ReValving System and Edwards Lifesciences SAPIEN™ THV obtained CE mark approval, newer transcatheter aortic valve devices have entered the clinical arena for assessment of safety and efficacy. Today, more than 10 start-up companies can be listed. Among these new comers are Direct Flow medical®, Sadra Medical®, and the PercValve® (Figure 7).
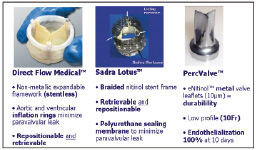
Figure 7. Second generation transcatheter aortic valve devices (Direct Flow Medical, Sadra Lotus heart valve, PercValve). In general, these devices aim to have a smaller profile and provide the options of “repositioning” and “retrievability.”
These newer devices offer several potential advantages compared to the existing technology.
Direct Flow
The Direct Flow Medical® device is a stentless prosthetic heart valve designed with an inflatable framework and conformable polyester fabric cuff that function to create a seal and minimise paravalvular aortic regurgitation37. A trileaflet bovine pericardium heart valve is attached to the frame. The valve is made functional after initial inflation of the ventricular ring with saline and contrast. Once satisfied with the position and functioning of the device, the saline and contrast is exchanged for a solidifying inflation media that hardens to form the permanent support structure. The device is designed in an hour-glass shape with independent inflatable aortic and ventricular rings that are suppose to encircle and “sandwich” the native aortic valve annulus for anchorage. The device is currently undergoing phase I clinical studies and approximately 30 patients have been implanted to date. Procedural success has been reported to be 80%38. These results compare favourably to the initial reports of procedural success using the 21 Fr and 18 Fr CoreValve ReValving System of 82% and 76%, respectively39.
Sadra Lotus
The Sadra Lotus valve prosthesis is a self-expanding, braided nitinol frame to which is attached a bovine pericardium heart valve in a trifoliate configuration. When stored within the 21 Fr delivery catheter, the prosthesis is in a longitudinally, elongated state and has low radial force. After crossing the native valve, retraction of the covering sheath of the prosthesis allows the nitinol structure to self-expand in the radial direction and foreshorten, thus increasing its radial force. The valve is linked to the delivery system by 3 wire cables attached to posts and locking mechanisms. The locking mechanisms maintain the valve in a compressed state. At this point, the device can be completely retrieved and then repositioned. If the positioning of the device is satisfactory, the prosthesis can be liberated from the delivery system via a releasing mechanism. A flexible sealing membrane (Adaptive Seal™) is attached to the lower portion of the prosthesis and functions to minimise paravalvular leakage. For purposes of clinical testing, the valve is currently available in a 23-mm size. The report of the first-in-man implantation has been published recently40.
PercValve
The PercValve makes use of nanotechnology to create metal valve leaflets composed of nitinol that are 10 um thickness. This technology constitutes a major revolution since for the first time, flexible metallic leaflet are used instead of biologic material. The durability of this material appears very promising. The extremely low thickness of the leaflets (10 um) and low profile of the device will allow to delivery the prosthesis using a 10 Fr delivery catheter system. Exciting data in vitro has shown that this highly biocompatible material facilitates the 100% endothelisation at 10 days. Only time will tell if this other dream will become true.
Conclusions
From the above discussion, we can reasonably conclude with the following. Firstly, the proof of concept of transcatheter aortic valve implantation has been validated. Secondly, transcatheter aortic valve implantation can be recommended for patients who are considered too high or prohibitive risk for surgical aortic valve replacement. It is likely that with increasing operator experience and CE mark approval of these devices, the medical community will soon expand this technology to involve patients with lower risk assessments, but also proceed with select randomised clinical trials as part of the technology maturation cycle. Newer generation devices will undoubtedly address some of the limitations of first generation devices. Finally, the cost-effectiveness analysis of this technology will precede its general adoption.

