Abstract
Aims: Abciximab provides dose-dependent antiplatelet and anti-inflammatory properties. No specifically designed prospective studies have addressed the role of intracoronary bolus administration of abciximab in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
Methods and results: In a prospective observational study 633 STEMI patients were enrolled. After coronary angiography the filtered abciximab bolus with a concentration of 2,000 µg/mL was administered intracoronary by manual injection, followed by an intravenous infusion for 12 hours. Primary outcome measure was the cumulative rate of major adverse cardiac events (MACE) at 30 days defined as death, myocardial infarction or urgent target vessel revascularisation. Bleeding events were classified according to TIMI criteria. Patients with failed thrombolysis, cardiopulmonary resuscitation, cardiogenic shock, advanced age, or renal failure were assigned to group II; patients without those criteria were assigned to group I and compared to previous populations with intravenous bolus administration. There were no safety issues. In group I MACE occurred in 3.6% (N=16/451), major TIMI bleed in 2.0%. MACE and bleeding events were significantly less frequent in group I compared to group II (both p<0.0001). Significant predictors for MACE in multivariate statistics were assignment to group II (OR 11.69; 95%-CI 6.26-21.83; p<0.0001), post PCI TIMI 0-2 flow (OR 3.87; 95%-CI 2.02-7.44; p<0.0001) and female gender (OR 2.05; 95%-CI 1.15-3.65, p=0.014).
Conclusions: The intracoronary administration of the abciximab bolus during PCI in STEMI patients is safe and associated with a low rate of MACE at 30 days.
Introduction
The glycoprotein IIb/IIIa inhibitor abciximab has been shown to reduce major adverse cardiac events (MACE) in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) with intravenous bolus application of abciximab.1-5 However, abciximab is rapidly bound with a high affinity to exposed glycoprotein IIb/IIIa receptors on the surface of circulating platelets, limiting the number of antibodies entering the culprit coronary vessel via the intravenous application route. Furthermore, the potential of abciximab for platelet disaggregation is dose-dependent.6 The intracoronary bolus administration provides high concentrations of abciximab during first-pass at the culprit lesion and in the distal bed of the culprit coronary vessel, facilitating dose-dependent antiplatelet and anti-inflammatory effects,7 which could translate into a further reduction of MACE. We have previously shown, in a retrospective analysis, a significant reduction of MACE with intracoronary compared to intravenous bolus application in acute coronary syndrome patients undergoing PCI.8 In patients without cardiogenic shock the 30-days MACE rate was 2.8% with intracoronary compared to 13.5% with intravenous administration of the abciximab bolus (p<0.001). No specifically designed prospective studies have addressed the role of intracoronary bolus administration of abciximab for primary PCI.
With STEMI patients directly admitted to the cath lab, the dose-dependent abilities of abciximab provided by intracoronary bolus administration could be superior compared to the standard intravenous approach due to a further reduction in MACE. We studied the impact of intracoronary bolus administration of abciximab in STEMI patients undergoing primary PCI in a large, prospective observational study.
Methods
Between January 2002 and September 2006 we included 633 STEMI patients undergoing PCI of a de novo lesion. For primary PCI, patients were directly admitted to the catheterisation lab by emergency medical services. Prior to admission patients received 500 mg bolus of acetylsalicylic acid and a heparin bolus of 5,000 IU intravenously by emergency medical services. Abciximab was filtered (MILLEX®GV Filter Unit 0.22 µm, Millipore, Carrigtwohill, Ireland). The bolus of abciximab (0.25 mg per kilogram of body weight) was given intracoronary in a concentration of 2,000 µg/mL by manual injection. In case of TIMI 2 or 3 flow the intracoronary bolus was given immediately after angiography of the culprit lesion, and in case of TIMI 0 or 1 flow after guidewire passage, or not later than after first balloon dilation. Subsequent 12-hour infusion of 0.125 µg per kilogram per minute (maximum, 10 µg per minute) was given intravenously. If necessary, additional boluses of heparin were administered to achieve an activated clotting time of 200-250 seconds. Stent implantation was performed, if the diameter of the infarct-related artery and the lesion were suitable for stenting. Different stent types were used including drug-eluting and non-drug eluting stents according to the physician’s discretion. Coronary angiograms were obtained according to standard acquisition guidelines. Coronary perfusion was graded according to the TIMI classification system. Clopidogrel was given after PCI, loading doses ranged from 300-600 mg. Sheaths were manually removed. Clopidogrel 75 mg once daily plus acetylsalicylic acid 100 mg per day were given throughout the study. The study was ethically approved and informed consent was obtained.
Clinical definitions
Cumulative major adverse cardiac events (MACE) were defined as a composite of any death, myocardial infarction (MI) or urgent target vessel revascularisation (TVR) within 30 days.1 MI was defined according to clinical symptoms and new electrocardiographic changes with a new elevation of creatine kinase or creatine kinase MB isoenzyme levels. Urgent TVR was defined as a repeated coronary revascularisation procedure or coronary-artery bypass grafting performed within 24 hours after a new ischaemic episode. Episodes of bleeding were defined according to the TIMI classification as major, minor or the combination as total.
Subgroups
To allow a comparison of MACE rates with historical groups receiving the abciximab bolus intravenously, the total population was separated in two prespecified subgroups. Patients with failed thrombolysis, cardiopulmonary resuscitation, cardiogenic shock, advanced age >80 years, or renal failure (creatinine more than 150 µmol/L) were assigned to group II. Patients without those criteria were included in group I. These subgroups were defined since patients with group II criteria are characterised by a high mortality due to non-cardiac reasons such as pulmonary or cerebral disorders and are usually not included in randomised clinical trials. Thus, patients in group I had comparable characteristics to patients treated in prior randomised clinical trials with intravenous bolus administration of abciximab.1-5
Statistical analysis
Continuous variables are presented as means±SD and categorical data as counts and percentages. Primary objective was to compare the MACE rates of group I to historical groups of patients in previous randomised clinical trials with intravenous administration of the abciximab bolus. Multivariate logistic regression analysis was performed for prediction of MACE. Following variables were included in the model: sex, group assignment, target vessel left anterior descending (LAD) artery, major TIMI bleed, post PCI TIMI 0-2 flow, diabetes mellitus. Statistics were calculated with Statistica (Version 7.1; StatSoft, Inc., Tulsa, OK, USA). Significance level was set at 5 percent.
Results
Study population
Baseline clinical and angiographic characteristics are shown in Table 1.
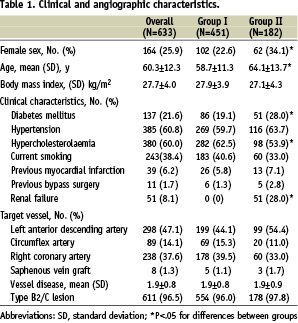
There were 451 patients included in group I. High-risk criteria qualifying for group II were present in 182 patients. Patients in group II were significantly more often female as well as diabetic patients and were significantly older compared to patients in group I. Criteria for assignment to group II were failed thrombolysis in 10.0% (N=63) patients, cardiopulmonary resuscitation 13.0% (N=82), cardiogenic shock 10.6% (N=67), advanced age 5.2% (N=33) or renal failure 8.1% (N=51). Thirty-four (66.7%) patients with renal failure had an additional criterion. Two or more criteria were seen in 51.1% (N=93/182) of patients in group II. Procedural characteristics are detailed in Table 2.
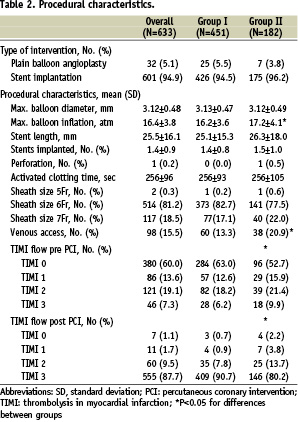
No difference was seen with respect to type of procedure, number of implanted stents, maximal balloon diameter, and maximal activated clotting time. Patients in group II received significantly more often an additional venous access and had a significantly lower final TIMI flow. There were no adverse events directly related to the administration of abciximab via the intracoronary route such as spasm, slow flow, perforation, or influence of haemodynamic situation.
Major adverse cardiac events
MACE at 30 days occurred in group I in 3.6% (N=16), based on death in 11 (2.4%), MI in four (0.9%), and urgent TVR in four (0.9%) patients. In group II MACE occurred in 31.9% (N=58). There were 56 (30.8%) deaths, three MI (1.7%) and three (1.7%) urgent TVR. MACE and death occurred significantly more often in group II compared to group I (p<0.0001). There was no statistical significant difference for MI or urgent TVR (p=0.41).
In patients with failed thrombolysis MACE occurred in 23.8% (N=15/63), cardiopulmonary resuscitation in 46.3% (N=38/82), cardiogenic shock in 56.7% (N=38/67), advanced age 21.2% (N=7/33) and renal failure in 66.7% (N=34/51). In patients with two or more criteria for group II MACE rate was 50.5% (N=47/93).
Bleeding events
In the total population major TIMI bleed occurred in 3.8% (N=24), minor in 4.4% (N=28) and total in 7.1% (N=52). In group II major, minor and total TIMI bleeding events occurred in 8.2% (N=15), 7.7% (N=14) and 15.9% (N=29). Values for group I were 2.0% (N=9), 3.1% (N=14) and 5.1% (N=23), respectively. Major, minor and total bleeding events were significantly less frequent in group I compared to group II (p=0.0002, p=0.011, p<0.0001).
Gender differences
The study population consisted of 469 men and 164 women. MACE rate was significantly higher in women (18.9%, N=31) compared to men (9.2%, N=43; p=0.0008). In group II MACE occurred significantly more often in women (43.6%, N=27/62) compared to men (25.8%, N=31/120; p=0.015). No statistical difference in MACE rates were present in group I with 3.4% (N=12/349) in men and 3.9% (N=4/102; p=0.82) in women. Total bleeding events were significantly more frequent in women with 12.2% (N=20) compared to men 6.8% (N=32; p=0.031).
Predictors of major adverse cardiac events
In multivariate analysis significant predictors for MACE were assignment to group II with an Odds Ration (OR) of 11.69 (95%-confidence interval 6.26-21.83, p<0.0001), post PCI TIMI 0-2 flow (OR 3.87, 95%-CI 2.02-7.44, p<0.0001) and female gender (OR 2.05, 95%-CI 1.15-3.65, p=0.014). Other parameters included in the model were non-LAD lesion (OR 1.21, 95%-CI 0.69-2.12, p=0.50), major TIMI bleed (OR 1.11, 95%-CI 0.35-3.49, p=0.86) and diabetes mellitus (OR 1.04 95%-CI 0.55-1.96, p=0.90).
Discussion
In this prospective observational study of STEMI patients with intracoronary bolus administration of abciximab during primary PCI, we noted a 30 days cumulative MACE incidence of 3.6%, which was lower than rates reported in randomised clinical trials (4.4-6.0%; Figure 1) with intravenous bolus application of abciximab in patients with comparable characteristics.1-5
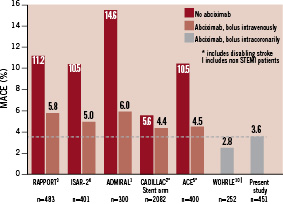
Figure 1. Incidence of MACE (death, MI, urgent TVR) at 30 days for randomised clinical trials with intravenous bolus administration of abciximab in primary PCI1-5 compared to intracoronary bolus administration (group I, including patients without failed thrombolysis, cardiopulmonary resuscitation, cardiogenic shock, advanced age >80 years, or renal failure). Both, in our previous retrospective analysis (patients without cardiogenic shock)7 and in this prospective observational study, MACE rates were lower with intracoronary compared to intravenous abciximab bolus administration in patient populations with comparable characteristics.
Group II, including high-risk patients, had significantly higher MACE as well as TIMI bleeding rates compared to group I. Criteria for group II were failed thrombolysis, cardiopulmonary resuscitation, cardiogenic shock, advanced age or renal failure. The high mortality in those patients is frequently related to pulmonary or cerebral disorders, wherefore those patients were usually not included in previous randomised clinical trials. Women were at a higher risk for MACE as well as bleeding events compared to men in the total population.
Abciximab was found to improve coronary and myocardial perfusion, recovery of the microvascular function as well as contractile function in the area at risk.1,4 Hence, abciximab reduced the occurrence of MACE (death, reinfarction, urgent TVR) in STEMI patients with intravenous bolus administration and obtained class IIa recommendation according to AHA/ACC guidelines9 as well as to ESC PCI guidelines.10 Nevertheless, there is a time-dependent11,12 as well as a dose-dependent ability of abciximab for platelet disaggregation.6 Abciximab concentrations seen after intravenous bolus application prevented further platelet aggregation in an in vitro study with adenosine-diphosphate stimulated platelets. However, with higher concentrations of abciximab up to 50 µg/mL the degree of platelet disaggregation was higher and faster.6 Intracoronary administration of abciximab bolus provides first-pass concentrations up to 2,000 µg/mL. Those high concentrations potentially facilitate dissolution of thrombus within the coronary artery and of thrombotic microemboli within the myocardium. Differently from the other glycoprotein IIb/IIIa inhibitors, abciximab binds with equivalent affinity to the glycoprotein IIb/IIIa receptor, the vitronectin receptor and the leukocyte aMB2 integrin.7 A higher local concentration of abciximab in the ischaemic area as provided with intracoronary bolus administration may also enhance the non-glycoprotein IIb/IIIa properties of abciximab. Those properties include anti-inflammatory effects from cross-reactivity with the leukocyte aMb2 integrin13 and the inhibition of the vitronectin receptors in the endothelial cells of the culprit vessel.14 By modulating inflammatory cell response due to non-glycoprotein IIb/IIIa effects in combination with inhibition of the glycoprotein IIb/IIIa receptor, abciximab may favourably influence post-ischaemic microvascular endothelial responses possibly leading to a reduced reperfusion injury.15,16 The more pronounced antiplatelet and anti-inflammatory effects at the culprit lesion and vessel by local intracoronary administration of abciximab may potentially translate in a further reduction of MACE compared to the standard intravenous approach.
The low 30-days MACE rate of 3.6% in 451 STEMI patients (group I) in the present prospective registry confirms the results of our retrospective analysis with a 2.8% MACE rate in 252 patients without cardiogenic shock.8 Both studies revealed lower MACE rates for the intracoronary bolus administration of abciximab than with intravenous bolus application in previously reported randomised clinical trials (Figure 1). Furthermore, the event rate was lower as compared to a recent randomised trial comparing early versus late administration of abciximab via the standard route of administration in STEMI patients undergoing primary PCI.11 210 patients were randomised to abciximab administration either in the emergency room or in the catheterisation laboratory after coronary angiography. ST-segment reduction, myocardial blush as well as left ventricular function recovery at one month were significantly better in the early compared to the late group. Nevertheless, cumulative MACE within one month was 8.6% in the late group as compared to 5.7% in the early group, both rates being higher compared to our 3.6% MACE rate with intracoronary bolus administration of abciximab (group I). The improved myocardial salvage with intracoronary compared to intravenous bolus administration of abciximab is supported by a recently presented study by Thiele et al.17 Consecutive STEMI patients with primary PCI were randomised to intracoronary (N=77) or intravenous (N=77) bolus administration of abciximab. With cardiovascular magnetic resonance imaging the infarct size (17.7±14.5% versus 24.7±13.9%, p=0.006) as well as microvascular obstruction (3.1±4.8% versus 5.5±6.4%, p=0.02) were seen to be significantly lower in the intracoronary group compared to the intravenous group. TIMI 2/3 flow (97.4% versus 96.0%) and TIMI myocardial perfusion grade 2/3 (90.9% versus 81.3%) were more frequent in the intracoronary group as compared to the intravenous group, correlating to a significantly better 90-min ST-segment resolution (76±23% versus 64±31%, p=0.007) in the intracoronary group as compared to the intravenous group. Furthermore, there was a trend regarding a lower MACE rate (death, reinfarction, new congestive heart failure, target vessel revascularisation) within 30 days in patients with intracoronary as compared to intravenous bolus administration of abciximab (p=0.06).
Although there is no randomised trial with a clinical endpoint comparing the intracoronary to the intravenous route of abciximab administration, the present data support the idea that the intracoronary bolus administration of abciximab may offer an optimised treatment strategy. This approach is limited to PCI centres with excellent logistics for patients with acute coronary syndromes. Such a management includes the direct admission of STEMI patients to the cath lab from medical emergency services, as well as a pre-hospital 12-lead ECG with a subsequent phone call activating the PCI staff. In centres without such capabilities, it is reasonable to start treatment with abciximab intravenously as early as possible before primary PCI in patients with STEMI.9
In conclusion, the incidence of MACE at 30 days after intracoronary bolus administration during PCI in STEMI patients was lower compared to previous studies including comparable patients with intravenous bolus application of abciximab. Our data support the hypothesis, that in PCI centres with direct admission of STEMI patients to the cath lab, the intracoronary bolus administration of abciximab may offer an optimised treatment strategy for patients undergoing primary PCI.

