Abstract
Aims: The objective of the study is to determine the demographics and the in-hospital outcome of diabetic and non-diabetic patients treated with percutaneous coronary interventions (PCI) in Europe, to report the type of equipment and technology used for PCI procedures in diabetics and to clarify whether the treatment of diabetic patients complies with current European Society of Cardiology (ESC) guidelines.
Methods and results: A total of 14,458 patients treated with PCI were enrolled from 29 member countries of the ESC between June 2005 and January 2006. Data were collected on patient characteristics and treatment, using new Cardiology Audit and Registration Data standards.
In total, 3,603 patients (24.9%) were diabetic. Diabetics were older, more often female and had a higher body mass index than non-diabetics. Diabetics had higher rates of hypercholesterolaemia and hypertension, while current smokers were more frequent in the non-diabetics. Diabetics also had significantly higher rates of previous cardiovascular events. Clopidogrel was administered only in 48.1% of diabetic patients before PCI, while IIb/IIIa inhibitors were 22.9% during PCI. At discharge, there was a major adjustment of treatment with increases in the use of Beta-blocker (80.4%), angiotensin converting enzyme inhibitor (ACEI, 71.3%) and statins (89.8%) compared with on admission (Beta-blocker 60.9%, ACEI 55.0%, statin 63.1%). In-hospital mortality was higher in diabetics (1.8% vs 1.2%) although the in-hospital MACCE rate was not significantly different (3.6% vs 3.0%, p=0.09).
Conclusions: Diabetic patients treated with PCI were older with more comorbidity. According to ESC guideline, the under-usage of clopidogrel, GP IIb/IIIa inhibitors should be improved. PCI is now taken as a good opportunity to adjust the use of appropriate medication.
Introduction
Diabetic patients are known to have an aggressive form of atherosclerosis with less favourable long-term survival following percutaneous coronary intervention (PCI) as compared to non-diabetic patients1-4,6-10. Currently, about 20% of patients with clinically established coronary artery disease have previously diagnosed diabetes and the prevalence of undiagnosed diabetes is also high11-12.
The European Heart Survey (EHS) Programme is aimed at providing up-to-date information on the current state of cardiovascular disease in Europe13,14. The most recent survey was performed in 2005-2006 for the percutaneous coronary intervention (PCI) registry database. General objectives of the survey included the determination of regional variations in the indications for revascularisation, and the assessment of the immediate, in-hospital outcome of patients assigned to different treatment strategies stratified by the clinical presentation and severity of coronary artery disease and patient comorbidities.
In this study, we investigated the EHS PCI database to: i) determine the demographics and the in-hospital outcome of diabetic and non-diabetic patients treated with PCI in Europe; ii) report the type of equipment and technology used for PCI procedures in diabetics and iii) clarify whether the treatment of diabetic patients complies to current European Society of Cardiology (ESC) guidelines5,15.
Subjects and methods
Study population
Between June 2005 and January 2006, 137 centres from 29 member countries of the European Society of Cardiology (ESC) enrolled 14,458 patients who underwent PCI. Data were collected on patient characteristics and treatment, using new Cardiology Audit and Registration Data standards (CARDS)16.
Definition
Previous history was obtained from the patients’ medical notes, referral letters or information from the patients’ family. Myocardial infarction was defined according to ESC/ACC guidelines5. MACCE was a combined endpoint of death, stroke or myocardial infarction. Major bleeding was defined as overt clinical bleeding associated with a drop in haemoglobin of greater than 5 g/dl or in haematocrit of 15%.
Statistical methods
Categorical data are presented as percentages. For continuous variables the mean ± standard deviation is shown. Subgroups were compared by Pearson chi-square test with respect to dichotomous or nominal categorical variables, by Mann-Whitney U-test with respect to ordinal and continuous variables, and by log-rank test with respect to the discrete durations of recommended antiplatelet therapy. Independent predictors of hospital mortality were analysed by stepwise selection in a logistic regression model using a significance level for entry of 0.1 and for stay of 0.15. Age, sex, ongoing ACS, elective PCI and the characteristics listed under past history relevant to CAD and risk factors in Table 1 were included as potential predictors.
A significance level of 0.05 was assumed and all p-values are the results of two-tailed tests. The statistical computations were performed using SAS, version 9.1 (Cary, NC, USA)
Results
In 14,458 enrolled patients, 3,603 patients (24.9%) were diabetic, in which 2.8% were newly diagnosed as diabetic, 15.3% were treated with dietary control, 58.2% with oral medication and 23.6% were on insulin. Prevalence of the diabetics in patients enrolled from each region was 19.4% from Northern Europe, 24.8% from Western Europe, 22.2% from Central Europe and 28.1% from Mediterranean. Patient characteristics, clinical presentation and angiographic results are presented in Table 1. Diabetics were older (65.7 vs. 62.9 years), less often male (67.7% vs. 76.9%) and had a higher body mass index than non-diabetics (29.0 vs. 27.4 kg/m2). Diabetics had higher rates of hypercholesterolaemia (69.2% vs. 61.2%) and hypertension (77.5% vs. 61.4%), while current smokers were more frequent in the non-diabetics (19.0% vs. 31.6%). Diabetics also had significantly higher rates of previous cardiovascular events. Regarding the clinical presentation, ACS with ongoing instability was less frequent (29.3% vs. 33.6%) and elective PCI was more frequent (44.8% vs. 42.0%) in diabetics. Normal left ventricle ejection fraction (>50%) was less frequent in diabetics than in non-diabetics (p<0.001). Angiographic results showed that 3-vessel disease was present in 29.1% of diabetics vs. 21.1% of non-diabetics (p<0.001).
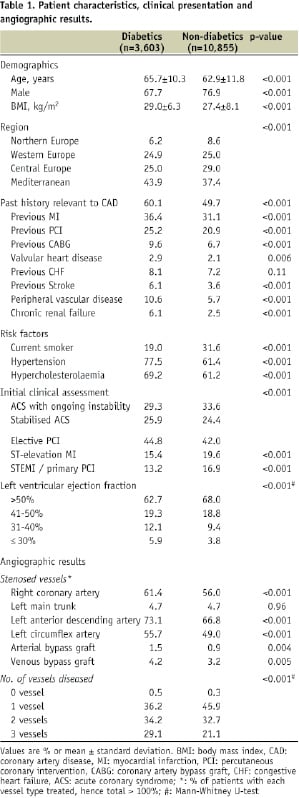
The use of medical therapy from the time of admission and discharge is presented in Figure 1.
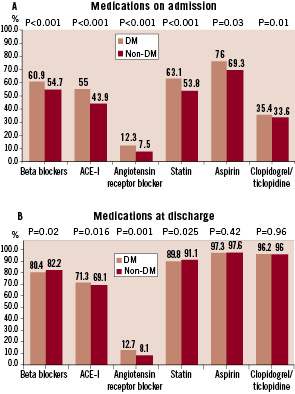
Figure 1 demonstrates medications on admission (A) and at discharge (B). P values are given for comparison between diabetics and non-diabetics. There was a major adjustment at discharge.
On admission, beta-blockers (BB), angiotensin converting enzyme inhibitors (ACE-I) and statins were used in half the patients, while diabetic patients had a strong tendency towards being prescribed more cardiac medications than non-diabetics. At discharge, there was a major adjustment of treatment with increases in the use of BB, ACE-I, angiotensin receptor blockers (ARB) and statins as well as antiplatelet agents. Approximately 80% of patients had beta-blockers and 90% of patients received a statin.
Figure 2 demonstrates the use of antiplatelet therapy before PCI.
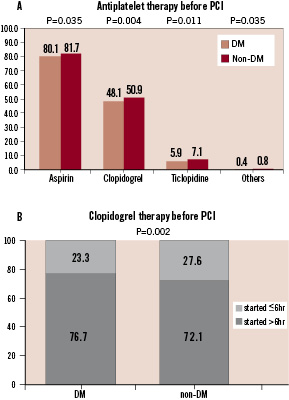
Figure 2 demonstrates anti-platelet therapy before PCI. Clopidogrel / Ticlopidine were given to only 54.0% of diabetics and 58.0% of non-diabetics (A). For the patients receiving clopidogrel, roughly one quarter started the agent less than 6 hours before the PCI procedure (B).
Aspirin was prescribed in almost 80% of patients, while clopidogrel was given to approximately half of the patients (Figure 2A). In roughly a quarter of those treated with clopidogrel, it was administered within 6 hours before PCI (Figure 2B). The recommended duration of dual antiplatelet therapy is presented in Figure 3.
Figure 3. Recommended duration of dual anti-platelet therapy is demonstrated in all patients and in the patients treated with DES.
In patients with drug-eluting stents, approximately 8 % of patients were recommended by physicians to have dual antiplatelet therapy for less than 6 months.
Tables 2 and 3 show the type of arterial access, lesion characteristics and devices used in PCI.
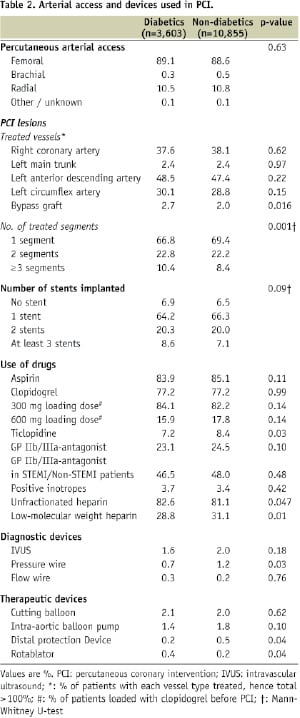
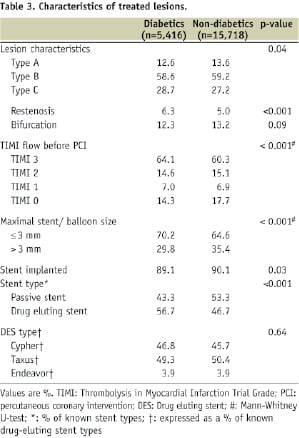
There were no differences in the route of arterial access between diabetics and non-diabetics; femoral access was the most frequently used. The left main trunk was treated at similar rates (2.4%) in both diabetics and non-diabetics. Type C and restenotic lesions were more frequently treated in diabetics than non-diabetics. The use of invasive diagnostic tools (intravascular ultrasound [1.6% vs. 2.0%], pressure wire [0.7% vs. 1.2%] and flow wire [0.3% vs. 0.2%]) was low in both groups. Diabetic patients had smaller calibre vessels treated: a maximum stent or balloon size ≤ 3 mm was more frequently used in diabetics. In diabetics, more PCI procedures involved the implantation of at least 3 stents than in non-diabetics. Bare-metal stents were more frequently used in non-diabetics (53.3% of stents implanted), while drug-eluting stents more in diabetics (56.7%).
Periprocedural complications and in-hospital outcome are shown in Table 4.
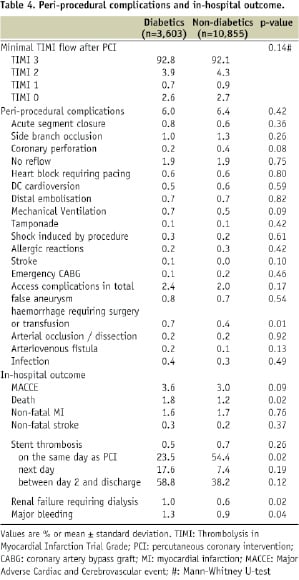
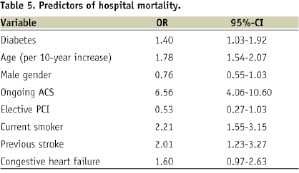
TIMI flow after PCI was not different between diabetics and non-diabetics. Overall, there were no differences in the rates of periprocedural complications between the 2 groups, except that haemorrhage requiring surgery or transfusion occurred more frequently in diabetics than in non-diabetics (0.7% vs. 0.4%, p=0.01). The rates of in-hospital death, renal failure and major bleeding were significantly higher in diabetic patients, however, the difference in the composite endpoint of death, non-fatal MI and non-fatal stroke was not statistically significant (3.6% vs. 3.0%, p=0.09). In addition, as a result of logistic regression analysis (Table 5), diabetes remained as an independent predictor of mortality. (Odds ratio 1.40, [95% CI: 1.03-1.92])
Discussion
In this survey of current practice of PCI in Europe, diabetics represented 24.9% out of 14,458 patients. This percentage is threefold higher than the prevalence of diabetics in Europe (7.8%, International Diabetes Federation). Although the diabetic prevalence does not differ from the previous revascularisation survey (23% in PCI treatment arm)13, the slightly higher prevalence of diabetes in this present survey may reflect the fact that the recruitment of the patients was weighted towards Mediterranean countries (39.0%) having a higher prevalence of diabetics of 28.1%.
The prevalence of smoking was lower in the diabetics (19.0% vs. 31.6%), while that of hypertension and hypercholesterolaemia was significantly higher in this group. This trend was also observed
in the EUROASPIRE II survey including 5,650 patients with history of hospitalisation for coronary heart disease, where diabetic patients were more obese and had higher systolic but lower diastolic blood pressure than non-diabetic patients and the prevalence of current smoking was lower in diabetes (17.3%) than non-diabetics (22.0%)18. The ESC guidelines on cardiovascular disease prevention in clinical practice in 200319 states that in patients with established CVD, lifestyle changes such as stopping smoking, making healthy food choices and increasing physical activity should be encouraged. To achieve these goals, lifestyle intervention programs focusing simultaneously on multiple preventable risk factors seemed to be most successful20,21.
The ESC guidelines generally pose more ambitious treatment goals for risk factors in diabetic patients than in non-diabetic patients24; <130/80 mmHg for blood pressure, <4.5 mmol/l for total cholesterol and <2.5 mmol/l for LDL cholesterol. In achieving these strict goals, hospitalisation for PCI seems to be a good opportunity to review and optimise medications. In the current survey, the usage of ACE-I, ARB, beta-blocker and statins before PCI in diabetics was 55.0%, 12.3%, 60.9% and 63.1%, whilst the use at discharge was 71.3%, 12.7%, 80.4% and 89.8%, respectively. From this point of view, there is improvement in this survey compared with the previous EHS survey on coronary revascularisation13 in which the usage of ACE-I, beta-blocker and statin at discharge remained 57%, 75% and 67%, respectively. This trend that an admission for revascularisation results in better use of guideline recommended medical treatment is also reported in other registries. In the REACH international outpatient-based registry including 40,450 patients with documented CAD, Steinberg et al reported that use of any lipid-lowering agent in those with previous CABG or PCI was 86% in CABG/PCI groups versus 70% in patients medically managed22. The possible reasons for this discrepancy are: i) treatment by cardiologists during and after revascularisation procedures increases the likelihood of the use of guideline-recommended therapies for CAD; ii) having a revascularisation procedure increases the patients’ and their physicians’ awareness of the CAD diagnosis, thus increasing prescriptions for and adherence to therapies23.
The ESC guidelines for diabetics with heart disease in 2007 state that in diabetic patients with CVD, statin therapy should be initiated regardless of baseline LDL cholesterol, with a treatment target of <1.8-2.0 mmol/l (Class I level B)24: they also indicate that screening for microalbuminuria and adequate blood pressure-lowering therapy using ACE-inhibitors and angiotensin receptor II blockers improve micro- and macrovascular morbidity in type 1 and type 2 diabetes (Class I level A). Although statin and ACE-I treatment at discharge in this survey is as high as 89.8 and 80.4%, there is room for improvement in pharmacological therapy in diabetic patients.
This survey demonstrated that diabetics had better baseline TIMI flow than non-diabetics. One of the possible causes is that ACS was less frequent in diabetics while elective PCI was more frequent. Interestingly, better baseline coronary flow in diabetics has previously been reported even in the setting of acute myocardial infarction. In 3,742 patients enrolled in the Primary Angioplasty in Myocardial Infarction (PAMI) studies, Harjai et al demonstrated that although diabetics had worse baseline clinical characteristics, longer pain onset-to-hospital arrival time and longer door-to balloon time, they nevertheless had better TIMI flow than non-diabetics25. They speculated that diabetics with ACS and poor TIMI flow may have died before reaching the hospital.
The ESC guidelines in 2005 stated that clopidogrel should have been orally administered at least 6 hours before PCI in stable CAD (Class IC recommendation), and that the duration of clopidogrel was recommended to be from 6 to 12 months after DES implantation15. The higher loading dose of 600 mg is recommended as pre-treatment for primary PCI, immediate PCI in NSTE-ACS or ad hoc PCI in stable CAD while the lower loading dose of 300 mg is recommended in stable CAD17. The current study demonstrates that there was an under-usage of clopidogrel before the PCI procedure. Clopidogrel or ticlopidine was administered to only roughly 50% of patients before PCI (Figure 2A), and approximately 25% of patients were given clopidogrel within the 6 hours preceding the PCI (Figure 2B). The duration of dual antiplatelet therapy was well abided: 92% of the patients having drug-eluting stents were recommended to have dual antiplatelet therapy for at least 6 months. The loading dose of 600 mg was administered in 15.9% of diabetics, while 300 mg was used in 84.1%. Recently, Angiolillo et al reported that high platelet reactivity in type 2 diabetics with coronary artery disease on chronic dual antiplatelet therapy is associated with a higher risk of long-term adverse cardiovascular events, suggesting the need for tailored antithrombotic drug regimens in high-risk patients26. The higher loading dose of 600 mg of clopidogrel has been proposed as a strategy to accelerate and enhance platelet inhibition compared with a loading dose of 300 mg and to improve short-term clinical outcomes compared with standard clopidogrel therapy27. An alternative approach will be the use of prasugrel, a new thienopyridine, with more potency and rapid onset, which has been evaluated in randomised trial with promising results28-30.
The previous ESC guidelines in 200515 stated that the use of GP IIb/IIIa inhibitors for PCI in stable angina should be considered on an elective basis in cases at high-risk of thrombotic complications (complex interventions, unstable lesions, as bail-out medication in case of threatening/ actual vessel closure, visible thrombus, or no/ slow-reflow phenomenon). In NSTE-ACS, GP IIb/IIIa inhibitors should be added only in high-risk patients, in whom an invasive strategy is planned (IC). In STEMI, guidelines recommend GP IIb/IIIa inhibitors use in all primary PCI (IIa A).
Now, the ESC guidelines for diabetics with heart disease renewed the statement that GP IIb/IIIa inhibitors are indicated also for elective PCI in a diabetic patient (Class IB)24. At the time this survey was performed, only the former guideline was published, so that only a small portion of diabetics received GP IIb/IIIa receptor blockers (7% before procedure, 22.9% during procedure). Broader use of GP IIb/IIIa is therefore expected in the future.
In this survey, 63.3% of diabetic patients treated with PCI had multivessel disease, although there is scant evidence demonstrating the benefit of PCI treatment over CABG treatment for multivessel disease. In a meta-analysis using pooled patient-level based 5-year follow-up data from ARTS, ERACI-II, MASS-II and SoS trials, the cumulative incidence of death, stroke or MI in the diabetic population was similar following PCI with stenting and CABG (21.4% vs. 20.9% respectively, p=0.9)31. However, the hazard ratio for repeat revascularisation in the diabetic subgroup was 0.19 (95% CI 0.12-0.30) due to a three-fold higher cumulative incidence of repeat revascularisation in the PCI group (29.7% vs. 9.2%; p<0.001)31. These results are still inconclusive because they are extracted results from diabetic subgroups without specifically targeting these populations. In addition, the above-mentioned trials were performed before the introduction of drug-eluting stents and accordingly comparison between contemporary CABG and PCI using drug-eluting stents is mandatory. The results of ongoing clinical randomised trials in diabetics such as the future Revascularisation Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM), The Coronary Artery Revascularisation in Diabetes (CARDia) trial and Coronary Artery Revascularisation in Diabetes (VA CARDs) is warranted.
The guidelines for diabetic/ pre-diabetic patients in 2007 state that drug-eluting stents should be used in all diabetic patients (Class IIa recommendation)24. In the survey, only 52.5% of diabetic patients were treated with drug-eluting stents. However, the benefit of DES in diabetics still needs further evaluation. In a recent meta-analysis of 18,023 patients by Stettler et al32, comparing the use of sirolimus-eluting stents, paclitaxel-eluting stents and bare-metal stents, there were no differences in myocardial infarction or death between the three stent groups in the diabetic population. The Ontario registry including 13,353 patients demonstrated a significant benefit of drug-eluting stents over bare-metal stents in reducing the need for repeat TVR especially in subgroups of patients with two or three risk factors for restenosis including diabetics, small diameter and long lesion33. For example, the number needed to treat (NNT) in diabetic patients with small diameter vessels (<3 mm) and long lesions (≥ 20 mm) was 10, while the NNT for those with large diameter vessels and short lesions (< 20 mm) but without diabetes was 167.
As for complications, major bleeding was more frequently observed in diabetics (1.3% vs 0.9%). Since major bleeding is known to be associated with poor outcome, special attention should be paid to this particular subset of patients. According to a meta-analysis on the prognostic impact of major bleeding in ACS by Hamon et al, major bleeding is a strong predictor of mortality (7.6-fold), myocardial infarction (3-fold) and stroke (5-fold)34. Of interest, in the meta-analysis, diabetes was not identified as a risk factor for bleeding.
The current analysis demonstrated that the rate of in-hospital stent thrombosis is comparable between diabetics and non-diabetics, while stent thrombosis in the same day is relatively lower in diabetics than non-diabetics. This may reflect the fact that the diabetics were less frequently treated in the setting of primary PCI than the non-diabetics, which has been identified as one of the independent predictors of the stent thrombosis35. Since late thrombosis is one of the concerns after DES implantation, data with longer-term follow-up in larger patients cohort is warranted.
Study limitations
This survey only involves voluntarily participating hospitals and the sample size only represents a small fraction of all patients treated with PCI throughout Europe in the study period. Of note, the number of diabetics patients treated with DES was about 1,900. Such a sample size represents a very small fraction of the overall diabetic patients treated with DES in Europe. Therefore the present analysis may not be reflective of the current European interventional practice in diabetes patients. However, as the PCI registry has collected data on large numbers of patients, we still believe that it provides a good estimate of disease demographics and therapy and will also enable individual clinicians to compare their own patient population and therapeutic strategies.
Conclusion
The current analysis revealed that diabetic patients treated with PCI were older with more comorbidity and more severe coronary disease than non-diabetics; they were subsequently associated with worse in-hospital mortality. The under-usage of clopidogrel, GP IIb/IIIa inhibitors should be improved. The use of appropriate medications was adjusted at discharge. Follow-up data in this registry will clarify whether optimal medical therapy is maintained. The small sample size is a limitation of this study.
Appendix: Organisation of the Survey
Percutaneous Coronary Intervention Expert Committee: Jean Marco (chairman), France; Anselm K. Gitt, Germany; Alec Vahanian, France; Franz Weidinger, Austria; William Wijns, Belgium; Uwe Zeymer, Sigmund Silber, Germany; Patrick Serruys, The Netherlands; Ricardo Seabra-Gomez, Portugal; Franz Eberli, Switzerland.
Euro Heart Survey Staff (European Heart House - France): Malika Manini, Operations Manager; Claire Bramley, Data Monitor; Valerie Laforest, Data Monitor; Charles Taylor, Database Administrator.
Statistical analysis centre (Institut für Herzinfarktforschung, Ludwigshafen, Germany): M. Hochadel (Statistician).
National Coordinators: Kurt Huber, Austria; Guy De Backer, Belgium; Vera Sirakova, Bulgaria; Roman Cerbak, Czech Republic; Per Thayssen, Denmark; Osama Abdel Aziz, Khalid Tammam, Egypt; Seppo Lehto, Finland; François Delahaye, France; Bondo Kobulia, Georgia; Uwe Zeymer, Germany; Dennis Cokkinos, Dimitrios Kremastinos, Greece; Kristof Karlocai, Hungary; Emer Shelley, Ireland; Shlomo Behar, Israel; Aldo Maggioni, Italy; Virginija Grabauskiene, Lithuania; Jaap Deckers, Netherlands; Inger Asmussen, Norway; Janina Stepinska, Poland; Lino Gonçalves, Candida Fonseca, Portugal; Vyacheslav Mareev, Russian Federation; Zorana Vasilijevic, Serbia & Montenegro; Igor Riecansky, Slovakia; Miran F. Kenda, Slovenia; Jose Luis Lopez-Sendon, Spain; Annika Rosengren, Sweden; Peter Buser, Switzerland; Tugrul Okay, Turkey; Oleg Sychov, Ukraine; Peter Schofield, United Kingdom.
There was no national coordinator in participating countries which are not mentioned in the above list.
Euro Heart Survey Board Committee: A.K. Gitt (chairman), Germany; L. Tavazzi, Italy; R. Seabra Gomes, Portugal; J. Marrugat de la Iglesia, Spain; L. Wallentin, Sweden; P. Kearney, Ireland; K. McGregor, France; M.L. Simoons, The Netherlands.
Industry Sponsor: Main sponsors: Boston Scientific, GlaxoSmithKline. Sponsors: Bristol-Myers Squibb, Eli Lilly.
List of Institutions: Belgium Working Group of Interventional Cardiology, Czech Society of Cardiology, Portuguese Cardiac Society,
Participating Centres, Investigators and Data Collection Officers:
Armenia: Kristine Margaryan, Shahen Khachatryan, Yerevan; Austria: Franz Weidinger, Jakob Doerler, Eva-Maria Stocker, Innsbruck; Johann Altenberger, Matthias Heigert, Max Pichler, Salzburg; Gunther Christ, Helmut Glogar, Irene Lang, Stefan Ingerle, Vienna; Belgium: Philip De Wilde, Michel de Marneffe, Bruxelles; M. Vrolix, Joseph Dens, Johan Van Lierde, Genk; X. De Wagter, Gent; Marc Carlier, Gilly; Antoon Weyne, Kortrijk; Victor Legrand, Pierre Doneux, Olivier Gach, Laurent Davin, Liège; Eric Mievis, Pierre-Emmanuel Massart, Namur; G. Holvoet, Oostende; Croatia: Lovel Giunio, Duska Glavas, Ivica Vukovic, Branimir Markovic, Darko Duplancic, F. Runjic, Spilt; Edvard Galic, Jure Mirat, Zagreb; Czech Republic: Petr Kala, Jiri Semenka, Ota Hlinomaz, Erik Petrikovits, Brno; Petr Widimsky, Petr Tousek, Prague; Ivo Varvarovsky, Pardubice; Denmark: P. Thayssen, Helle Cappelen, Odense; Steffen Helqvist, Henning Kelbaek, Erik Jorgensen, T. Engstrom, Kari Saunamaki, Jens Kastrup, Peter Clemmensen, H. Hansen, Copenhagen; Egypt: Magid Al Abbadi, Khalid Tammam, Hany Abdel Razek, Gamal Aboul el Nasr, Hany Ragi, Basam Ibrihim, Basam Zarif, Naha el Banhawy, Khalid Sorour, Mohamed Abdel Meguid, Ahmed Mahrous, Cairo; Khalid Ahmed Al Khashab, Fayoum; Ahmed Abd Elmoniem, Metwaly El Emry, Amr El Naggar, Benha; Aly Saad, Zagazig; Estonia: Peep Laanmets, Jyri Voitk, Piia Lutter, Sigrid Jarvekulg, Mati Jalakas, Julia Reinmets, Toomas Marandi, Margus Peeba, Tarmo Serka, Tallin; Finland: Mikko Syvannne, Esa Kaihovirta, Helsinki; Kari Korpilahti, Mari-Anne Vaittinen, Vaasa; France: Jean-Pierre Bassand, Denis Pales Espinosa, Besancon; Yves Cottin, Isabelle Lhuillier, Philippe Buffet, L. Lorgis, Dijon; Jacques Machecourt, Bernard Bertrand, Delphine Serrano, Grenoble; Jean-Louis Bonnet, Marseille; Philippe Gabriel Steg, Jean-Michel Juliard, Reza Farnoud, Paris; Nicolas Delarche, Pau; Jean Marco, Frederic Petit, Bruno Farah, Didier Carrie, Michel Galinier, Jacques Puel, Jacqueline Cahuzac, Jerome Roncalli, Stephane Tauzin, Meyer Elbaz, Toulouse; Germany: Volker Schachinger, Frankfurt; Anselm Gitt, Uwe Zeymer, Ludwigshafen am Rhein; Ralf Zahn, Boris Fraiture, Siegberto Haetinger, Nurnberg; H. Klepzig, E. Girth, A. Hauber, Offenbach; Christian Firschke, Jochen Widmaier, Florian Hofbauer, Stefan Huttl, Pfaffenhofen; Udo Sechtem, Ulli Parade, Stuttgart; Georg Linnartz, Viersen; Greece: D. Cokkinos, Stalikas, Andrianidis, Natassa Tsiavou, Athens; Georgios Papaioannou, Efthymios Deliargyris, Maroussi Attikis; Dimitrios Alexopoulos, Periklis Davlouros, Patras; Dimitrios Tsikaderis, Petros Dardas, Nikolaos Mezilis, Thessaloniki; Hungary: Edes Istvan, Bakos Zoltan, Debrecen; Israel: Yoav Turgeman, Suleiman Khaled, Alexander Feldman, Afula; Jamal Jafari, Iliya Manevich, Ashkelon; Carlos Cafri, Reuben Ilia, Akram Abu-Ful, Sergei Yaroslavslev, Jean Mark Wainstain, Gabriel Rosenchtein, Beer Sheva; Ricardo Krakover, Beer Yakov; David Halon (& the CV Medecine department), Luis Gruberg, Walter Markiewicz, Ehud Grenadier, Monther Boulos, Ariel Roguin, Arthur Kerner, Shlomo Amikam, Margalit Ben-Tzvi, Jeremy Rezmovitz, Haifa; Morris Mosseri, Haim Lotan, Boris Varshizky, Hisham Nassar, Haim Daninberg, David Rot, Tedi Vais, Jesaia Benhorin, Andre Keren, Aharon Medina, Zahi Huri, Jerusalem; Simcha Brandis, Guy Schoenmann, Netanya; Ran Kornowski, Abed Assali, Shmuel Fuch, David Hasdai, David Brosh, Rehavia, Offer Sela, Igal Teplitski, Petach Tikva; Oded Eisenberg, Rehovot; Shmuel Banai, Ariel Finkelstein, Tel-Aviv; Yonathan Hasin, Muein Aboud, Menachem Nahir, Daud Qarwani, Genem Diab, Tiberias; Italy: Luigi Meloni, Giorgio Lai, M. Cadeddu, R.Pirisi, Cagliari; Francesco Bonechi, Franco Nassi, Massimiliano Nieri, Andrea Taiti, Alessandra Naldoni, Francesco Calabro, Fucecchio; Felice Achilli, Stefano Maggiolini, Luigi Piatti, Gianluca Tiberti, Piero Addamiano, Lecco; Sergio Berti, Marcello Ravani, Cataldo Palmieri, Giuseppe Trianni, Simona Cardullo, Massa; Angelo Cioppa, Paolo Rubino, Vittorio Ambrosini, L. Salemme, G. Sorropago, T. Tesorio, Mercogliano; Giuseppe Geraci, Modena; Filippo Scalise, Simone Mazzeti, Carla Auguadro, Monza; Giovanni Esposito, Napoli; Guido Canali, Negrar; Maria Enrica Caccia, Chiara Ruggieri, Bertola Benedetta, Novara; Nicoletta de Cesare, M. De Benedictis, Tiziana Coco, Sabrina Manzotti, Osio Sotto Fraz. Zingonia; Paolo Marraccini, Pisa; Alessandro Danesi, R. Ricci, A. Ferraironi, E. Olivieri, A., Chiera, Roma; Stefano Garducci, Daniela Grasseli, Vimercate; Ireland: Peter Kearney, Eugene McFadden, Noel Cahill, Cork; Martin Quinn, Peter Crean, Eileen Caroll, David Foley, Stephen O’Connor, Rory O’Hanlon, Bernadette Lynch, Srah O’Donnell, James Roy, Darragh O’Brien, Dublin; Latvia: Asnate Krastina, Andrejs Erglis, Riga; Lebanon: Samih Lawand, Beirut; Poland: Waldemar Dorniak, Jacek Klaudel, Krzysztof Pawlowski, Wojciech Trenkner, Gdansk; Marianna Janion, Marcin Sadowski, Agnieszka Janion-Sadowska, Kielce; Izabella Skorupa, Leszek Bystryk, Lublin; Adam Kern, Olsztyn; Bartlomiej Janiak, Roman Szelemej, Walbrzych; Witold Ruzyllo, Adam Witkowski, Tomasz Deptuch, Renata Maczynska-Mazuruk, Warsaw; Andrezj Budaj, Kokowicz Lewandowski Cegieska, Grzegorz Opolski, Joanna Wilczyska, Marek Roik, Janusz Kochman, Warszawa; Portugal: Dinis Martins, Acores; Isabel Maria Fernandes Joao Goncalves, Helder Pereira, Almada; Henrique Faria, Joao Calisto, Lino Goncalves, Victor Matos, Antonio Leitao-Marques, M. Costa, H. Oliveira, P. Mota, Coimbra; Walter Santos, Victor Brandao, Faro; Graca Caires, Bruno Silva, Funchal; Rui Campante Teles, Manuel Almeida, Pedro Goncalves, Luis Raposo, Luis Mourao, Luis Bernardes, Paulo G. Pedro, Rui Ferreira, Rui Conduto, Jorge Quininha, Lino Patricio, Duarte Cacela, Jose Maria Goncalves, Lidia de Sousa, Manuela Adao, Lisbon; Henrique Cyrne Carvalho, Helia Romeira, Jose Paulino Sousa, Jose Manuel Mota Garcia, J. Carlos Silva, Domingos Magalhaes, Porto; Ricardo Santos, Setubal; Pedro Gama Mendes, Joao Pipa, Luis Nunes, Pedro Ferreira, Viseu; Romania: Dragos Vinereanu, Cristian Udroiu, Nicolae Florescu, Octavian Parvu, Claudiu Stoicescu, Maria Dorobantu, Serban Mihai Balanescu, Rodica Niculescu, Lucian Calmac, M. Marinescu, Bucharest; Dan Olinic, Mihai Ober, Calin Homorodean, Claudia Budurea, Adrian Hij, Florin Anton, Cluj-Napoca; Florin Ortan, Ciprian Suciu, Mihai Ursu, Costica Baba, Targu-Mures; Stefan Iosif Dragulescu, Lucian Petrescu, Milovan Slovenski, Dan Gavrilescu, Cristian Dina, Bogdan Mut, Timisoara; Serbia & Montenegro: Rade Babic, Mirko Colic, Dragan Topic, Belgrad; Spain: Joan Bassaganyas Vilarrasa, Marti Puigfel Pont, Rafael Masia Martorell, Izabella Rohlfs, Girona; Rafael Melgares Moreno, Granada; Maria Irurita, Juncal Irurita, Las Palmas de Gran Canaria; Carlos Escobar Cervantes, Madrid; Teresa Galvan, Jimenez Navarro, Dominguez Franco, Malaga; Ignacio Santos Rodriguez, Victor Hogo Ramirez, Salamanca; Francisco Fernandes-Aviles, Ana Revilla, Pedro Mota, Valladolid; Switzerland: Nicholas Masson, Virginie Dupertuis, Genève; Tunisia: Salem Kachboura, Ariana; Turkey: Atila Iyisoy, Ankara; Mustafa Kemal Erol, Erzurum; Zeki Ongen, Erhan Babalik, Mehmet Oskan, Nihal Ozdemir, Ali Oto, Kudret Aytemir, Bunyemin Yavuz, Istanbul; Mahmut Sahin, Kenan Durna, Samsun; Vedat Aytekin, Cemsit Demiroglu, Murat Gulbaran, Saide Aytekin, Alp Burak Catakoglu, Burak Ozme, Gokmen Gemici, Hasan Feray, Sisli; United Kingdom: P.M. Schofield, S. Kahn, S. Clarke, Heather Millington, Cambridge; Carlo Di Mario, Debra Dempster, London; Robert Anthony Henderson, Jane Burton, Della Falcon-Lang, Nottingham.

