Abstract
Aims: To develop a clinical prediction rule based on 3D reconstruction of coronary arteries that would prospectively identify lesions that are difficult to cross and could benefit from magnetic navigation.
Methods and results: The coronary anatomy of a cohort of 120 lesions that had undergone percutaneous coronary intervention (derivation set) was analysed using 3D reconstruction for vessel and lesion characteristics. The crossing time was the total clock time to reach a satisfactory distal position after leaving the guiding catheter. Multivariable logistic regression and linear shrinkage with bootstrapping were used to develop a clinical prediction rule that dichotomised cases into easy or difficult (prolonged crossing time).
A value of 6 was the best cut-off value. This clinical prediction rule was applied to a second independent cohort of patients (validation set) where crossing time was measured. The bootstrapped c-statistic of the model was 0.82 indicating excellent discrimination.
Conclusions: 3D reconstruction helped to develop a simple, accurate clinical prediction rule to identify difficult cases for conventional wires and in whom magnetic navigation may be preferable.
Introduction
Currently the majority of percutaneous coronary intervention (PCI) procedures are performed satisfactorily with conventional wires. However, there is a proportion of PCI procedures where difficulty, or sometimes failure, occurs. As equipment improves and PCI extends into more difficult territories, e.g. smaller, more tortuous, heavily calcified and more distal lesions, there will be an increasing challenge to manual dexterity. The recently introduced magnetic navigation system (MNS) from Stereotaxis uses a magnetic field to precisely steer the wire that can take direction from a 3D model of the vessel created from angiographic images, and may be of benefit in such problematic procedures.1 Improved wire delivery may lead to reduced procedural time and complications.2-4
However, there is no specific method of identifying those cases where passing the lesion with a wire, or ‘crossing’, is difficult. The currently available range of lesion classification systems5-9 is aimed at predicting complications and these have relatively modest accuracies. We have investigated a simple, specific, measurable and crucial part of the PCI procedure, the time to pass the wire from the guiding catheter to a position distal to the culprit lesion.
Such prediction may have a number of major benefits. First, better guidance for operators in the assessment of the difficulty of a particular case may help better risk-benefit decision-making. Second, it may allow selection of patients who have difficult morphology for an alternate technique such as magnetic navigation. A clinical prediction rule (CPR) may be a useful method of ultimately comparing magnetic navigation or other navigation technologies to conventional wire manipulation.
The aims of this study were two-fold. First, the development of a simple CPR to predict difficult lesions to cross with a conventional wire. Second, the validation of this CPR in lesions that had undergone angioplasty.
Methods
Patient selection
Derivation set
The crossing times of a cohort of 425 consecutive lesions that had elective and semi-urgent PCI performed with a conventional wire
at a single centre were noted. Lesions that were excluded from analysis consisted of occluded vessels where a reconstruction could not be produced due to inadequate vessel flow (TIMI flow of 2 or less) such as primary PCI, acute coronary syndrome (ACS), and chronic total occlusion (CTO). ACS patients were generally not excluded.
In addition, lesions where special techniques had been used, such as biplane angiography, or magnetic navigation, were excluded. Several strategies were undertaken in order to reduce possible bias. The crossing times of the patients were consecutively measured and the times were analysed to show the boundaries of the tertiles (Figure 1). The patient data were left unsorted when separated into the three groups of 40 lesions. The films of each of these groups were examined consecutively and the inclusion for analysis was determined by a coin toss. The person analysing the films and performing reconstructions was completely blinded to the crossing time. The derivation set comprised of these three groups and the CPR was developed from this.
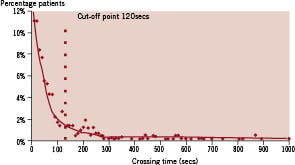
Figure 1. Graph showing distribution of the total population.
Validation set
The CPR was validated in a second cohort of 415 lesions, the validation set. The same exclusion criteria and strategies were used as in the derivation set. Sixteen lesions were randomly selected from each tertile of the crossing times for further analysis. In order to blind the analysis, the analyst had no knowledge of the results of the crossing time.
Magnetic navigation system
The MNS (Navigant, Stereotaxis, St Louis, MO, USA) has dedicated 3D reconstruction software (CardiOp-B, Paieon Medical Inc., Rosh Ha’ayin, Israel) that produces a virtual model of the entire vessel2,10. With the reconstruction package it is possible to take 2 angiographic views and identify the vessel edges. The reconstructions were performed from suitable films where 2 separate films at suitable angulations (of >40°). As the angles of the image intensifier and the position of the table are known, it is possible to triangulate the position of each point of the vessel and make a 3D reconstruction of the vessel that is volume-rendered (Figure 2). All measurements can be made from diagnostic angiography films of suitable quality. There were 3, 2 and 6 films that were not suitable for analysis in the shortest, middle and longest tertiles respectively –the average inability to produce a reconstruction was therefore approximately 10% of the total, but with a trend increasing in more complex morphology.
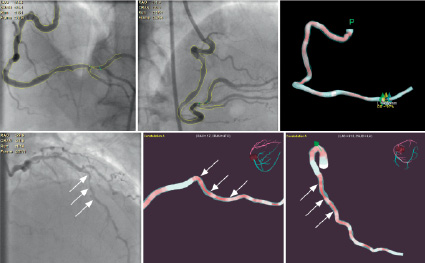
Figure 2. The upper panels: The left 2 panels show separate angiographic angulations with edges detected and the right panel shows a 2D image of the 3D reconstruction. The lower panels: The left panel shows the angiographic view of the LAD, the middle and right panels show the reconstruction in different views with the angles shown by the 3D reconstruction arrowed.
To produce as accurate and reproducible reconstructions as possible, the images were routinely taken in diastole (on or just before the QRS complex). The software has functions that allow measurement of length, diameter and area stenosis with respect to the calculated reference diameter at that point. The model can then be imported into the Navigant programme where the angulation of each bend can be measured. The bends were assessed visually and then measured to see whether they fitted the following definition. A bend was considered to be an angulation of 45° within 10 vessel diameters (for the purpose of this analysis a standard vessel diameter was considered to be 3 mm therefore a bend had to occur within 30 mm).
Definition of crossing time
A prolonged crossing time was defined as the absolute time (in seconds) from the initial entry of the wire into the coronary system, out of the guiding catheter, until crossing and reaching a suitable position distal to the target culprit lesion in the coronary artery. The crossing time includes the time required for any and all procedural manoeuvres once the wire has initially left the tip of the guiding catheter, such as the time required to remove and re-shape the conventional wire, re-engagement of the catheter should prolapse occur, or to pass a balloon for support, and implies the placement of the wire in a satisfactory final position for device delivery.
This was a registry where no special techniques of passing a wire were used, and therefore the operators should have been performing normally. A total of 9 interventional cardiologists were involved in these procedures. While a competitive element may have occurred within an operator’s mind, the data once collected was only available to one person. This data was not, and has never been, shown in a fashion that would compare one operator with another and while operators may well have tried to perform their best, this is felt to be an advantage with the ‘best’ crossing times recorded.
The relevant hypothesised anatomical factors encountered with conventional angioplasty guidewires can be divided into vessel and lesion characteristics, see appendix 1. We imposed an arbitrary maximum to each factor in order to prevent any specific factor from dominating the grading system.
Vessel characteristics
Number of bends before the lesion (Vb) (Figure 3)
Each bend causes deformation of the wire resulting in friction. A greater number of bends leads to increased friction resulting in more difficultly in manipulating the wire. The maximum score is 5.
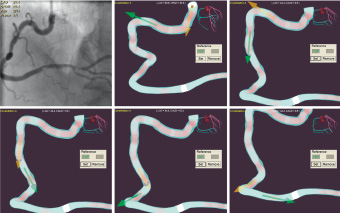
Figure 3. Identification of the bends in a vessel and measurement of each angle (possible in the Navigant program).
The total angulation of the 5 worst bends (Vta) (Figure 3)
The greater the cumulative angle, the more the wire is deformed along its course. This results in more friction and produces increased difficulty during manipulation. This has been dichotomised to above 9= 1) and below 45° (= 0). The maximum score is 1.
End-to-end length from the ostium to the lesion (Vl) (Figure 4)
The more distal the lesion is from the ostium, then the greater the chance of encountering problematic bends that impair manipulation, and also the longer the time required to physically pass the wire. This is divided into shorter than 50 mm (=0), between 50 and 100 mm (=1) and greater then 100 mm (=2). The maximum is 2.
Figure 4. The measurement of the length from the vessel ostium to the lesion in CardiOp-B (green square indicates proximal marking point and blue square indicates distal marking point).
Vessel calcification (Vc)
Calcium may increase friction as the vessel becomes more rigid and less deformable and does not conform to the wire. The frictive effect of a specific angle will be accentuated if deformation cannot occur. The complete angiographic absence of calcium scores 0, mild calcification is 1 and severe calcification is 2. The maximum is 2.
Visible side-branches before lesion (Vsb) (Figure 5)
Visible side-branches result in a greater chance of taking a wrong bend. This is dichotomised to 0 or 1.
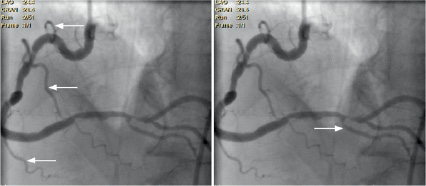
Figure 5. The left panel shows visible side-branches (arrowed) greater then 1 mm before the lesion with the right panel showing a branch just before the lesion (within 10 mm).
Lesion factors
Luminal lesion characteristics such as complex and intricate 3D form or tight lesions with little space for manoeuvre affect how quickly and easily a wire can pass. Navigation through complex, intricate, and/or tight lesions may involve a variety of angles that cannot be easily be traversed by a conventional guide-wire with a fixed bend as it has a specific angle of approach.
Lesion characteristics
Side-branches within 10 mm (Lbb) (Figure 5)
Side-branches within 10 mm of the lesion increase difficultly because, on the approach to a lesion finer control is necessary, the fixed wire-tip angle needed for bends now may have more of a predisposition for side-branches. This is dichotomised to 0 or 1.
Side-branches in lesion (Lsb)
When the angulated wire tip has to pass through a stenosis, there is a greater chance that the tip will be pushed against the wall and this increases the chance of selecting side-branches. This is dichotomised to 0 or 1.
Lesion angulation (La)
The lesion angulation will affect the friction of the wire passing through the lesion. This is dichotomised to 0 for <45° or 1 for > 45°.
Lesion calcification (Lc)
Calcium makes the lesion rigid and reduces deformability, therefore producing more friction. This is dichotomised to 0 or 1.
Lesion length (Ll) (Figure 6)
Longer lesions produce more friction on the wire. This factor has been divided into <10 mm (=0), between 10 and 20 mm (=1) and greater then 20 mm (=2). The maximum is 2.
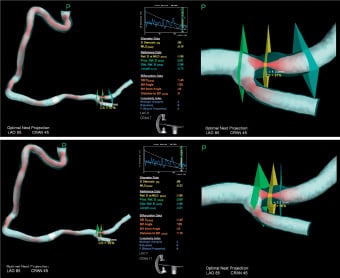
Figure 6. The top panels show the measurement of the lesion length (green square indicates proximal marking point and blue square indicates distal marking point). The lower panel shows the segment greater than 80% measured in CardiOp-B (green square indicates proximal marking point and blue square indicates distal marking point).
Length of severe (greater than 80% area) stenosis (Lss) (Figure 6)
Tighter lesions have greater contact with the wire and this magnifies the effect of bends leading to a greater chance of the wire being forced against the wall. This is dichotomised to into 0 for <3 mm and 1 for >3 mm.
Statistical analysis
A prolonged crossing time, Pct, was dichotomised with an arbitrary cut-off value of 120 seconds based on the observed crossing times (Figure 1). By the design of the study, one-third of the lesions had the presence of a long crossing time and two-thirds had a normal crossing time.
Statistical modelling
The association between each prognostic factor and the presence or absence of a long crossing time was studied by univariate logistic regression analysis. We applied a stepwise backward selection process (p=0.100) to select the predictors for the final model.
A key problem of regression modelling in small data sets is that the regression coefficients are overestimated for predictive purposes. We calculated a linear shrinkage factor for the regression coefficients with bootstrapping.11 The difference between performance in the bootstrap samples and the original sample is an estimate of the optimism in the apparent performance (miscalibration). This difference is averaged to obtain a stable estimate of the optimism. ‘Shrunk’ coefficients were calculated by multiplication of the standard coefficients with the shrinkage factor, which might take values between 0 and 1.
The final model was transformed into a CPR. Regression coefficients were rounded to the nearest integer. A score was obtained for each individual lesion by assigning points for each variable and adding the results.
Predictive performance
The prognostic ability of the model, i.e. the power to discriminate between subjects with and without a long crossing time, was estimated with c-statistic. The c-statistic, provides a quantitative summary of the discriminative ability of a predictive model. A value of 0.5 indicates that the model does not have any discriminatory ability, and a value of 1.0 represents perfect discrimination. Calibration refers to whether prediction models agree with the observed probabilities and was assessed with the Hosmer-Lemeshow statistic.
Statistical analyses were performed with SPSS and S-plus software (MathSoft, Inc., Seattle WA, USA: version 2000).
Results
Mean age was 65 years and 74% were male. All baseline characteristics in the derivation set and the validation set were generally similar although there were more unstable patients in the validation subset (Table 1). The vessel location of the culprit lesion is shown in Table 2.
There was a spread of values for the crossing times showing that 15% of lesions could still not be crossed at 5 minutes (310 s) and 10% by 8 min (480 s) (Figure 1). The CPR was derived after applying logistic regression to the sample from the derivation group (results of univariate and multivariate analysis shown in Tables 3 and 4) to provide a relative estimate of crossing complexity:
Pct=1*Vb+1*(Vl=1)+2*(Vl=2)+2*Vc+1*Lbb)+1*(Ll=1)+2*(Ll=2)
(N.B. abbreviations refer to morphological factors, see appendix 1)
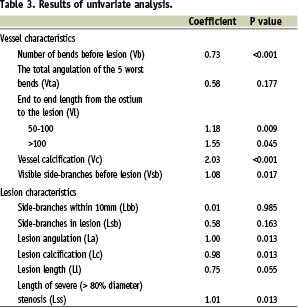
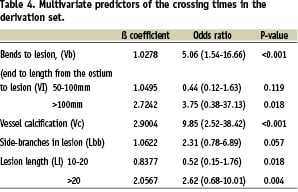
This dichotomises cases into easy and difficult (prolonged crossing time). Pct is an integer and a value of 6 was found to be the best cut-off value. The c-index of the standard model was 0.88 and 0.83 for the shrunk model used for the CPR. Calibration with the Hosmer-Lemeshow test gave a non-significant p-value. This CPR was then applied to the results from the validation cohort. The cstatistic derived from this group was 0.82 showing good discrimination.
Discussion
We have developed a clinical prediction rule (CPR) that examines the whole vessel that is undergoing PCI and is able to predict which vessels will require more time to cross. The CPR integrates the use of a 3D reconstruction developed from a combination of lesion and vessel characteristics to give a relative estimate of crossing complexity. It will allow an assessment of the importance of physical factors on wire passage and whether difficult wire passage is related to increased complication rates. This is directly applicable to the decision process of selecting a patient for a magnetically navigated procedure.
This system is fundamentally different from previous classification systems that were developed for the prediction of procedural success and complications. The ACC/AHA guidelines for lesion grading were developed in 1988,5 modified in 1990,6 and use 11 simple angiographic factors to help take into account lesion complexity in the comparison of the outcomes of PCI. Further systems, such as the SCAI,7 the Mayo Risk Score8 or the SYNTAX score,9 were subsequently developed in order to improve this predictive ability further. However, the discriminatory power was lower than expected.6,12 A number of possible reasons may help to explain this shortcoming. First, previous grading systems use a number of coronary factors that have generally been simple, angiographically-defined lesion characteristics. Use of such simple lesion characteristics may underestimate both the lesion complexity itself and also the importance of the rest of the vessel in successfully crossing with a wire. Second, while these factors have a great deal of merit in their simplicity, they are visual estimates from angiographic images and these are known to have poor inter-observer reproducibility.13-15 Third, some systems include clinical factors, either as an endpoint or in their grading, that are frequently complex and multi-factorial. Difficulty in crossing an individual coronary lesion, especially if wire passage itself results in a clinical complication such as dissection, may only be indirectly related to a poor clinical state of an acutely sick patient or to the pre-existing clinical diseases. Conversely, a poor clinical state or pre-existing disease may be more closely associated with a complication than the adverse coronary anatomy. Therefore the use of clinical factors, either the pre-existing clinical diseases or the resultant clinical complication, may obscure a clear answer.
A number of factors have been investigated in previous studies. These indicate that increasing complexity of a lesion is associated with an increasing incidence of complications7,16 and that some anatomical factors of the vessel, such as tortuosity, lesion length or angulation, are associated with decreased success of stent passage.17-19 The introduction and use of new 3D imaging technology enables a more complete anatomical analysis of the vessel and lesion in a new way. In order to give the clearest idea of factors that might influence passage of the wire we analysed the entire vessel as well as the target lesion. This approach has not been used before.
All the measurements required for the CPR can be obtained from adequate diagnostic angiography films. The CPR can be applied in a matter of minutes and is therefore applicable in general practice. This system is completely independent of the clinical factors that, while important in the broad clinical outcome, are either only loosely related, or even irrelevant, to the passage of a wire through a coronary artery. The analysis aimed to reflect these factors by defining the anatomy of both the coronary vessel and lesion. There are a number of advantages of this system. First, it uses a 3D reconstruction to identify and measure specific vessel factors and to give hard numbers to predict a single, clearly definable endpoint. Second, it examines the anatomy of the entire vessel, a factor that is clearly crucial to the delivery of a wire. Third, it is independent of the clinical factors that obscure the importance of physical factors. Fourth, it has clear applicability in choosing patients who might benefit from a magnetically navigated procedure.
There are a number of limitations. Currently the reconstruction is time-consuming and can take up to 30 minutes to complete. The production of the reconstruction requires angiography of a suitable standard and is not always possible from standard views due to foreshortening or overlapping. The reconstruction that is produced is static and the impact of movement on the measured factors, such as angulation, is unknown. The use of the software does require some practice and some manual correction is often needed. This was deliberately kept to a minimum.
After analysis some factors were not incorporated into the CPR. There may be a variety of reasons for this. Some factors occurred infrequently, such as the presence of a stent. The infrequent occurrence therefore may not reflect the unimportance of the factor but the effect of a small sample size e.g. few patients with stents. Another possible reason for a particular factor not showing significance is the variability of the factor itself. Side-branches may be cannulated without intention during procedures but this may only occur with side-branches that come off at a certain angle and not all as measured in this analysis. Therefore, in general, these factors may play some role, but this may only occur in specific circumstances, or occasionally, and will need further investigation to identify any additional benefit. More generally, there was unselected use of wires and no selection of operators. The limitations of this model therefore also relate to individual operators, techniques and equipment. There were a number of operators and types of wires and this, together with no selection of the target vessel segment, may have led to heterogeneity. In addition, this study examines only one specific aspect of the PCI and deliberately does not address other necessary elements such as the passage and deployment of the balloon and stent. Lastly, this study was performed at a university specialist tertiary referral unit and the case mix might be different to that seen elsewhere.
Despite such limitations, the system could prove useful in application. The ability to predict difficult cases may allow consideration for alternative strategies. In this context, the ability of the MNS to precisely direct the tip of a wire has a clear potential for improving the treatment of patients identified in this was as suggested in the study in this issue of EuroIntervention20. However the system could potentially be used in other ways. As the number of MNS in an institution is normally limited to one, reconstruction could be performed on selected subsets of patients. Identification of the patients who would most benefit would be not only beneficial for patients but would maximise efficiency. Alternately, an automated system would also allow all patients to be screened in addition to possibly improving reproducibility.
This CPR will be valuable in several ways. First, to identify which anatomical factors are predominant by prospectively identifying difficult cases. Second, this may be a crucial tool in deciding whether a patient should undergo a conventional procedure or rather will benefit from magnetic navigation.
Conclusion
Previous classification systems have been of significant clinical use but relatively modest predictive ability due to a reliance on few lesion features and no vessel features, inclusion of clinical features with indirect relevance to the procedure, and the use of broad clinical endpoints. This study shows an accurate clinical prediction rule can be developed that uses the 3D reconstruction software of the magnetic navigation system to aid identification of difficult lesions for guide-wire placement on the basis of specific coronary analysis. It provides only five measurable, anatomical variables and has a simple but crucial endpoint. This clinical prediction rule is a good predictor of prolonged crossing time (cstatistic of 0.82) and may provide a more accurate and prospective method of predicting difficult procedures in which magnetic navigation might be a better option than the conventional techniques.
Acknowledgement
The authors are grateful to Joep Maeijer for help with preparation of the images.
Appendix


