Abstract
Aims: Long lesions and complex vessel anatomy frequently require the use of overlapping stents to treat a lesion. The purpose of this study was to evaluate the long-term effects of overlapping the Axxess Biolimus A9™ eluting stent (BES) with two of the most commonly used, commercially available drug eluting stents. These stents were compared to BxVelocity bare metal (BMS) stents in a porcine coronary stent-injury model.
Methods and results: Nineteen juvenile farm swine, 25-35 kg in weight, 3-6 months in age were utilised. Each animal received an Axxess™ stent to their coronary artery as permitted by the individual animal’s anatomy. A second stent, either a Cypher™, sirolimus eluting stent (SES) or, a Taxus™, paclitaxel eluting stent (PES), or a BxVelocity™ bare metal stent (BMS) were implanted in an overlapped fashion. The animals were then followed for either 28 or 180 days as specified by a randomisation scheme. At the end of each follow-up period, they were euthenised, and the vessels containing the overlapping stents were harvested, processed into histological sections, and analysed. Compared to bare metal stents, overlapped segments using DES exhibited delayed vascular healing compared to both the proximal and distal non-overlap sites at each of the follow-up time point. Overall, in the non-overlap stent segments, SES induced significantly more inflammation and neointimal hyperplasia compared to PES and BMS.
Conclusions: In this study of BMS and two different types of DES overlapped with the Axxess Biolimus A9™ eluting stent, we found that while there was a delay in the degree of vascular healing with DES compared to BMS, the specific type of DES that was overlapped with BES did not affect the behaviour of the overlap zone in terms of most of the histomorphometric measures at 28 or 180 days. This was true whether the stent was drug eluting or bare metal. More inflammation with delayed healing was seen in the SES compared to PES and BMS.
Introduction
Complex anatomic situations frequently necessitate the use of multiple overlapping stents for complete lesion revascularisation. Currently, usual practice is to have some degree of stent overlap in order to assure that a gap in drug coverage does not promote restenosis in the non-overlap segment.
The Axxess™ stent is a Biolimus A9™ eluting, (DEVAX, Inc. Irvine, CA, USA licensed the drug Biolimus A9™ and a bioabsorbable polymer coating from Occam International [Menlo Park, CA, USA]), self-expanding nickel titanium alloy stent designed specifically for use in coronary bifurcation lesions.
The Axxess™ stent is completely coated with a permanent Paralene C layer. The Biolimus A9™ is applied to the surface of the stent using a bioabsorbable polylactic acid (PLA) based polymer carrier.
Similar to sirolimus, Biolimus A9™ binds to the cytosolic immunophylin FKBP1; thus, both sirolimus and biolimus inhibit growth factor-driven cell proliferation, including that of T-cells and vascular smooth muscle cells. Biolimus also possess increased lipophilicity compared to sirolimus, and this may enhance cellular uptake and migration within the cellular membrane.
Axxess Biolimus A9™ eluting stent is self-expanding in order to optimise deployment at a vessel bifurcation. The conical shape of the stent conforms to the bifurcation anatomy and provides full access to both branches for additional interventional procedures. We recently described the long term effects of Biolimus A9™ eluting self expanding Axxess™ stent in a porcine coronary artery model1.
Commercially available stents eluting sirolimus (Cypher™, Cordis, Johnson & Johnson, Warren, NJ, USA) and paclitaxel (Taxus™, Boston Scientific, Natick, MA, USA) have been shown to reduce neointimal proliferation compared to bare metal stents2-5. Both of these DES stent platforms use polymer coatings over stainless steel stents to deliver the drug. BxVelocity™ is a bare metal stent (Cordis, Johnson & Johnson, Warren, NJ, USA). The pathological effects of overlapping these types of stents with the Axxess Biolimus A9™ eluting stent are unknown.
Thus, the purpose of this study was to assess the safety and potential efficacy of overlapping the most commercially available DES and bare metal controls with the Axxess Biolimus A9™ eluting stent at 28 and 180 days follow-up.
Methods
Animal husbandry
All aspects of the study were performed and audited in accordance with the required elements of 21 CFR Part 58, with good laboratory practices (GLP) requirements. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas.
Stent implantation
Nineteen juvenile farm swine, 25-35 kg in weight, 3-6 months in age underwent placement of an Axxess™ stent into the each coronary artery as permitted by coronary anatomy (Figure 1, zone 1). A second stent was implanted distal to the Axxess™ stent, with a target of a 5 mm overlap which was measured by quantitative coronary angiogram (QCA) (Figure 1, zone 2). The second stent was either a Cypher™, sirolimus eluting stent (SES), a Taxus™, paclitaxel eluting stent (PES), or a BxVelocity™, bare metal stent (BMS).
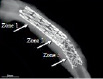
Figure 1. Proximal AXXESS biolimus A9™ eluting stent with a distally placed balloon expendable stent with an overlap segment.
All animals received antiplatelet therapy with 325 mg aspirin and 75 mg clopidogrel daily commencing three days prior to the procedure. They were fasting at least 12 hours prior to surgery. After sedation with a xylazine/ketamine cocktail intramuscularly (17 mg/cc & 83 mg/cc respectively dosed at 3cc/22.5 kg), each animal was intubated and placed on maintenance anaesthesia with oxygen and isoflurane 1%-2%. A carotid artery cut-down was performed and an 8 Fr sheath was placed in the carotid artery. Heparin was administered (200-300 u/kg, IV) and it was re-administered at 1/2 dose if necessary at 30 minutes following initial dose depending on ACT results.
An 8 Fr guide catheter of appropriate shape was placed into the left or right coronary artery ostium using fluoroscopic guidance. Intracoronary nitroglycerine (200 µg) was administered prior to baseline cine-angiography. Cine-angiography was performed in two orthogonal views. Baseline QCA was performed for assessment of target vessel diameter. An approximately 20 mm length segment was identified where the vessel diameter measured approximately 3.0 mm. All stents were individually packaged, coded with a serial number on the packing label and ETO sterilised. A prespecified implantation protocol was used to balance the number of stent types used in the study. The total amount of Biolimus drug in the self-expanding nitinol stents was 308 µg (308µg/stent or 22 µg/mm).
A 0.014” guidewire was placed in the target vessel. The Axxess™ stents were deployed first without prior balloon angioplasty. Axxess™ stent was 14 mm in length. The purposely overlap stent (Either Cypher™, Taxus™ or bare metal) was then placed distal to the Axxess™ stent, with a target of 5 mm of overlap (This is longer than what is in the usual clinical practice). SES and BMS stents were 13 mm and PES stent was 12 mm in length. All stents were 3 mm in diameter. Nominal pressures, per manufacturer’s recommendations, were used for deployment of second stents; 10 Atm. for PES and 11 Atm. for BMS and SES with post-dilation at the overlap region at 12 Atm. for 15 seconds. Individual stents were deployed at their respective nominal pressures (PES at 10 Atm., rest at 11 Atm., 30 seconds balloon inflation). Post-dilation of overlapped segment (5 mm target) with a PTCA catheter (size nearest to 0.25 mm) at a 1.1:1 stent: artery ratio was done at 12 Atm. Stents were placed in arterial locations without major braches. Intracoronary nitroglycerin (200 µg) was administered immediately before and after each stent deployment to prevent coronary vasospasm. A final cine-angiogram of the treated vessel was obtained in the same views as baseline.
After the initial procedure animals were recovered and returned to the animal care facility where they received antiplatelet therapy with 325 mg of aspirin along with 75 mg of clopidogrel daily during the study period. There were no operative deaths. The animals were placed into 28 or 180 days follow-up periods. At the end of each study period, follow-up angiography was performed for all animals prior to sacrifice. Follow-up coronary angiograms were performed on the stent treated arteries in the same views as in the baseline procedure to document patency and position of the overlapping stents. After completion of the angiograms, the animal was euthanised with intravenous Euthasol® at 1 cc/10 lbs.
Pathologic evaluation
After euthanasia, the hearts were harvested immediately and rinsed with 0.9% normal saline, with care taken not to compress the stents. The coronary arteries were perfusion fixed in situ at 100 mmHg mean pressure using 10% buffered formalin for an hour. The hearts were stored in fixative solution and forwarded to a pathology core laboratory, at the Biomedical Research Foundation of South Texas, Dr. Fermin Tio, for vessel dissection and histology.
Upon arrival at the pathology laboratory, the hearts were photographed and radiographed (Faxitron). The coronary arteries were then dissected from the heart and coronary-stented segments were dehydrated in graded series of alcohol and processed for plastic embedding, staining and morphometric analysis of six sections from the proximal through the distal margin of the stent. The specimens were embedded in methyl methacrylate and sections (4 µm) were obtained with a Beuchler isomet saw (Beucher, Evanston, IL, USA). Multiple cross sections were obtained from the proximal to the distal end of the vessel, polished, mounted on a glass slide and stained with hematoxylin and eosin and metochromatic stains. Fibrin was identified on hematoxylin and eosin-stained sections and with Castair’s histochemical stain. Luna stains were performed to identify eosinophils. The sections were scored for the technical quality and eight good quality sections were selected for analysis from the proximal non-stented segment, proximal stent segment, three from the overlap segment, two distal segments, and one distal non-stented segment.
Area measurements of the artery, stent/IEM, and lumen were obtained using a Nikon compound microscope. Vessel images were directly downloaded and digitised with an Intel based computer through a drawing tube attachment and a pin-point LED lit puck. Morphometric measurements for vessel area, stent area, and lumen area were made using Sigma-scan scientific measurement software (version 3.9, SPSS , San Rafael, CA, USA). Morphologic analysis of injury, inflammation, endothelisation, fibrin, and smooth muscle content were completed using published data2,6. Intimal area, percent stenosis, and other ratios and dimensions were calculated based upon direct measurements. Neointimal area was calculated as the difference between the lumen area and the stent area. Percent area stenosis was calculated using the formula: [neointimal area/stent area] x 100. In the overlap zone, the outer stent was used to measure stent/IEL area, and thus the neointimal area included the overlapped stent. The sections were evaluated under 4x objective and scored for mural injury, inflammation, vascularisation, intimal fibrin, intimal smooth muscle, adventitial fibrosis and endothelisation.
Quantitative coronary angiography
All the digital cine-angiograms were analysed using an automated edge-detection algorithm (CAAS-II, Pie Medical, the Netherlands). The analysis was blinded to the second overlap stent type. The proximal stent was always an Axxess™ stent. Image calibration was performed using contrast-filled catheters as the reference standard. The balloon to artery ratio (diameter of balloon/reference vessel diameter at baseline), late loss (difference in minimal luminal diameter (MLD) from post-intervention to follow-up), and late loss index and diameter stenosis (percent stenosis in the stent at follow-up) were calculated for each stent. Angiography after implantation demonstrated that all stents were patent without side-branch occlusion, intraluminal filling defects or dissections.
Statistical analysis
All the data from the analysis of the stented sections were sorted by treatment group and divided into follow-up cohorts (28, 180 days). The data was then stratified by level and grouped according to zone (Axxess™ stent, overlap, or distal stent). Different zones were tested for normality and then zone differences within groups and among groups were tested using 1-way ANOVA (Tukey-Kramer). Data are expressed as mean ±S.D. unless otherwise stated.
If the data in the zone distribution was not normal, or if there were significant differences among section level data within a group, the differences were tested with Kruskal-Wallis. For non-parametric data, scores were assigned to each of the three sections within the stented segment and the median value as the scores for the stent. Differences were tested with the Kruskal-Wallis. These analysis were performed with the Statistical Analysis Software version 9.1 (SAS Institute inc., Cary, NC, USA).
Results
Nineteen juvenile swine (25 to 35 Kg) underwent placement of 90 stents in 51 vessels. Each vessel received an Axxess™ stent. There were 17 each of the Cypher, Taxus, and bare metal stents paired with the Axxess™ stent. A computer randomisation was used to assure a diverse order and distribution of the stents among the different coronary arteries. Average ratios of stent to artery were similar among groups, as was the total length of the stented arterial segments. The mean length of overlap was 3.48+1.39 mm without significant differences in length of overlap between experimental stent groups. There were no procedural deaths. Follow-up angiogram at euthanasia showed preservation of stent overlap with no evidence of edge effects, stent thrombosis, and aneurysm formation during the entire study. In one 28 day animal, a BMS placed in the right coronary artery was not overlapped due to migration distal to the Axxess™ stent during the procedure, so this vessel was excluded from the analysis. An additional five vessels were lost due to two post-procedure deaths in the 180 day group. There were three post-procedure deaths in the study. Two pigs died from 180 day group. One at 52 days likely from cardiac cause, in whom stents were implanted in all three vessels. Post-mortem pathology demonstrated significant stenosis in all three coronary vessels, including a 98% area stenosis in the left anterior descending artery (LAD). The second animal from the same group died at 126 days, in which PES was implanted in the LAD and an SES in the Left Circumflex coronary artery (LCX). Examination of this pig’s heart revealed autolysis with epicardial fibrosis and evidence of an old myocardial infarction, but acute infarction was not present. Thus, the total number of vessels utilised in final analysis was therefore 48 vessels.
Overall, at the end of the 28 and 180 day follow-up, the overlap zone stent area was consistently larger than either the proximal or distal single stent zone, had consistently higher injury and inflammation scores and greater intimal tissue response as measured by either neointimal area or by% area stenosis. Despite these factors, the lumen size remained equal or larger than the distal single stented segment. The overlap lumen area was equal or smaller than the proximal stented segment, a finding consistent with a vessel tapering from proximal to distal over a 25 mm length. Notably, all of the groups except the overlap zone, demonstrated a complete endothelial cell layer by 28 day.
Quantitative coronary angiography
The baseline vessel diameter was similar across all three stent groups (average baseline mean luminal diameter: 3.26+0.21). The balloon to artery ratio was similar for all groups, approximately 1.1 to 1.
The 28 day outcome by quantitative coronary angiography (QCA) showed significant increase in MLD in the PES group compared to SES and BMS (MLD: 3.05+0.28, 2.49+0.23, 2.73+0.64, P<0.001).
At 180 days, follow-up angiograms were obtained for all animals and all stents were patent but, because of the large body size of the animals and poor quality of the angiograms, no QCA analysis could be performed.
28 day histopathology
Nine hearts containing 22 double stented coronary arteries were analysed for the 28 day time-point. There were eight stents each from the SES and PES study groups, and six from the BMS group. External gross examination of the hearts showed no gross abnormalities along the stented coronary arteries. There was no epicardial haemorrhage or aneurysm, other than that in the animal which died at 27 days, showing an acute 0.4cm sub-epicardial haemorrhage in the anterior and posterior walls.
Table 1 and Figures 2-3 report the averages and standard deviations for the key histomorphometric data arranged by the treatment groups. There were significant differences among the mean values at the proximal, overlap, and distal segments. The PES group showed more complete stent expansion as reflected in the larger stent area. The neointimal hyperplasia was also lowest in the PES group at 28 day follow-up. This combination of full stent expansion and suppressed intimal tissue growth resulted in a larger lumen at 28 day follow-up in the PES group compared to either BMS or SES. The luminal patency expressed as% diameter stenosis, was similar across all groups and was below the threshold for restenosis (< 50%). The BMS and SES groups were almost identical at 34%, while the PES was somewhat less at 20% diameter stenosis. The injury and inflammation scores were consistently less than 1.5, ranging from 0.56 for PES and 0.76 for SES. The ratio of intimal SMC to intimal fibrin is a reflection of intimal maturation. Mature intima has a full complement of intimal SMC with only a trace residual intimal fibrin, while less mature young intima will have low intimal smooth muscle score with more prominent deposits of intimal fibrin. There was a slight delay in intimal maturation in both the SES and BMS groups, and more apparent delay in the PES group. All groups showed full endothelial coverage of the luminal surface at 28 days.
The effect of the overlap compared to the single stent zones was evaluated by comparing the proximal (Zone 1) and distal (Zone 3) segments to the overlapped segments within each group. In all three groups, the overlap segment (Zone 2) had the largest average stent diameter compared to the proximal and distal segments. Thus, the expansion of the second overlapping stent within the Axxess™ stent had the effect of further expanding the Axxess™ stent diameter in the overlap zone. The increase in diameter was 0.1 mm for the both the BMS and SES. The increase in diameter was 0.3 mm in the Express stent (PES). The increased diameter in the PES group occurred despite a lower injury score in the overlap and distal stent zones.
The overlap segment typically had more injury than the single stent zone for all groups, but the difference was not significant. Inflammation was significantly higher in the overlap zones in both DES groups compared to the non-overlapped single stent zone, but this was not seen in the BMS group. There was more intimal tissue within the overlap segments than within either single stent segments. However, the overlap segment, the intimal area measurement included the inner stent. In all cases, the overlap segment lumen area was greater than the adjacent distal segment. This is consistent with a tapering vessel. The intimal area for the similar class of drugs (Biolimus A9™, sirolimus) resulted in very similar amounts of neointimal tissue in the proximal and distal segments, as seen in the SES group.
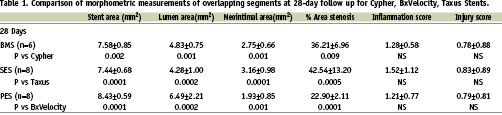
Figure 2. Neointimal hyperplasia at the overlap segments for each stent type.
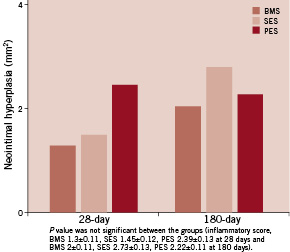
Figure 3. Inflammatory score.
When comparing the Axxess™ stent overlap segment between the PES or SES stents, no differences in stent, lumen and intimal area,% stenosis, inflammation score, or injury score were observed. The PES group overlap zone had the largest stent area and lowest intimal area, resulting in the largest lumen and least amount of luminal stenosis. In the overlap zone, Inflammation and injury scores did not differ across the study groups within the overlap zone. The PES also had significantly larger stent areas compared to SES and lowest intimal area in the distal non overlap stent segment. In the overlap zone, the SES group had more intimal tissue and a smaller lumen than the BMS group. As with the inter-group comparisons, the lumen area in the overlap zone was larger than in the distal zone. The mechanism of smaller lumen area and more neointimal hyperplasia in the SES compared to PES remains enigmatic.
Representative histologic sections for the 28 day study groups are presented in Figure 4. The greater expansion of the PES stent is apparent by the larger stent diameter in the overlap and distal stent segments, by the close apposition of the stent struts at the level of the overlap, and in the distal segment.
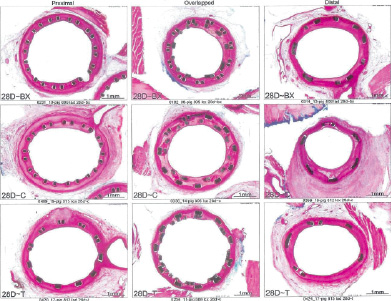
Figure 4. Images representing average luminal stenosis for proximal, overlapped and distal stented sections for each treatment group at 28 days.
180 day histopathology
At 180 days, seven of nine treated animals surviving through the 180 day period were sacrificed and 18 stented vessels were harvested. Gross examination of the hearts showed no abnormalities along the coronary arteries. The data in general showed a reduction in luminal stenosis and inflammation at 180 days compared to the 28 day time period. The injury scores for all three groups were high, ranging from 2.2 to 2.87. The luminal stenosis for the BMS and PES was about 44%, while the stenosis for the SES group was significantly higher at 52% (P=0.016). The lumen area in the BMS and PES groups were slightly higher than the SES group, although the difference was not significant. In this time interval, the overall stent area was about the same. As demonstrated at 28 days, smaller intimal areas correlated with larger lumens. Inflammation scores were high in all groups, with the BMS group again showing the lowest score at 1.38 (P<0.0001). Inflammation continued to correlate with increased luminal stenosis. The nature of the inflammatory reaction at this time-point was generally granulamatous in type with multinucleated foreign body giant cell formation. There was no change in intimal maturation as the intimal fibrin score remained low against a normal complement of intimal smooth muscle. There was less adventitial fibrosis at 180 days vs. 28 days for all groups. Full endothelial cell coverage was maintained in all treatment groups. At 180 days, the intimal proliferation and percent stenosis remained highest in the overlapped area. As in earlier time periods, part of this difference was due to the presence of the second stent in the overlap segment. The lumen area was greatest in the proximal segment, but the difference was less pronounced. The lumen area in the overlap was not significantly different than the distal segment in any group. There was not a large difference in the injury score between the overlapped and single stent segments in the SES and PES groups. In the BMS group, there was significantly less injury in the single stent segments compared to the overlap. The finding of minimal inflammation in the distal segment may be related to the low injury score. The proximal stented segment is composed of the Axxess™ stent in all three treatment groups. This serves as a “control” segment and did not exhibit any there differences in stent area, lumen area, or percent stenosis across all groups. There was no difference in neointimal proliferation in the overlap segment, although there was a trend toward less intimal area and percent stenosis in the PES group. The stenosis in the distal non-overlap segment was consistent across all groups, with the lowest observed value in the BMS group (28%) and highest in the SES group (46%). The inflammation and injury scores were high in all groups. In the overlapped segment, all three groups showed similar luminal stenosis ranging from 51 to 61%. The SES group was consistently the highest in terms of inflammation, injury, intimal area and% stenosis. The most intimal area was in the proximal Axxess segment, but this was not reflected in lumen area due to the larger stent area of the Axxess™ stent. In general, the BMS and PES had the least injury and inflammatory reaction at this time period. At 180 days, all groups showed less luminal stenosis, inflammation, adventitial fibrosis than the 28 day period. The overlap regions continued to have more intimal tissue, but as with the 28 day time period, the lumen size was not compromised, since the stent area was larger at this level as well. The SES group showed the most intimal tissue growth and inflammation and the BMS group the least, followed closely by the PES group. Representative histologic sections for the 180 day study groups are presented in Figure 5.
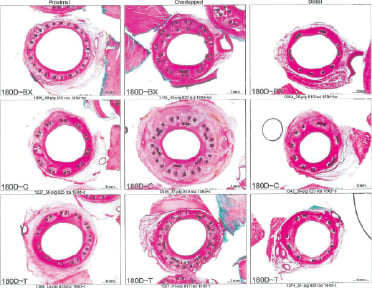
Figure 5. Images representing average luminal stenosis for proximal, overlapped and distal stented sections for each treatment group at 180 days.
Discussion
Complex coronary lesions and clinical scenarios often require deployment of multiple, frequently overlapping stents. As an example, 33% of patients enrolled in the Sirolimus-Eluting Balloon Expandable Stents in the Treatment of Patients with De Novo Coronary Artery Lesions (SIRIUS) and European multicentre, randomised, double-blind study of long atherosclerotic lesions in small coronary arteries (E-SIRIUS) were treated with overlapping stents compared with 29% in TAXUS-IV7-9. Despite the relatively common use of this technique in clinical trials and daily practice, the long term effects of this technique on the arterial wall pathology remains mostly unknown. Although, DES allow higher local drug concentration at lesion sites while avoiding systemic toxicity, they raise considerable concerns over local toxicity as drug and/or polymer concentrations and are likely to be significantly higher at sites of stent overlap10. Prior animal studies of sirolimus- and paclitaxel-eluting stents show a dose-dependent drug response of incomplete arterial healing characterised by persistent fibrin deposition, medial necrosis; increased intimal inflammation2,4. This paper is the first to study the long term effects of most commonly used overlapping DES and novel Biolimus A9™ eluting Axxess™ stents in a porcine coronary artery model.
In our study, both SES and PES showed delayed arterial healing at the non-overlapping segments, which is in agreement with other published reports of sirolimus- and paclitaxel-eluting stents. In this investigation, we demonstrate more significant inflammation, injury score, fibrin deposition, delayed endothelisation, as well as more significant neointimal hyperplasia at the overlapping segments. This finding, suggests that, in the porcine model, local arterial toxicity may potentially develop when surrounding tissue is exposed to the levels of drug and/or polymer present when stents are overlapped. The inflammatory response differed between DES types. Overlapping SES induced more fibrin and eosinophils compared to PES. Moreover, although both SES and PES evoked a giant cell response near stent struts, it was greater with SES. Incomplete healing however in both DES was principally characterised by persistent fibrin and inflammatory cell infiltrate with eosinophils for PES and giant cells for SES. Although no significant differences were found in cell proliferation or intimal cell density suggestive of increased apoptosis, at or near stent struts was significantly lower in both 28-day follow-ups with PES.
These findings are consistent with previous published studies of single DES implants that demonstrated minimal delayed healing at 28 days, whereas the late follow up studies showed neointimal healing to be nearly complete with mild inflammation. The results from our study of the overlap site are reason for concern in that we found an increase in local arterial toxicity characterised by delayed endothelisation, persistent fibrin deposition, and enhanced inflammation at the sites of overlap with SES. The lack of exaggerated eosinophilic response in the overlapping segments of the BMS control allows one to hypothesise that the overlapping layers of polymer and/or drug may be responsible for the excessive inflammation. This is consistent with the findings of Suzuki et al in that PEVA/PMBA non-erodible polymers used in the Cypher stent demonstrated an increase in arterial inflammation as polymer load was increased2.
Another key finding from this study in the porcine animal model is that the PES group consistently resulted in less neointimal hyperplasia than the SES group, both in the overlap or the distal zone, suggesting superior suppression of neointimal hyperplasia for the paclitaxel based stent. Given the fact that both DES were implanted at similar high pressures, the radial strength of PES (Express) stent is greater than the SES (BxVelocity) stent. This may account for the internal elastic lamina differences between two groups. This observation is not consistent with data reported in the large, randomised clinical trials, comparing PES and SES in humans, where SES consistently demonstrated superior suppression of intimal tissue growth compared to PES or differences in native vessels versus atherosclerotic vessels. The typical findings are that SES results in approximately half the amount of in-stent intimal hyperplasia compared to paclitaxel, as measured by in-stent late loss (QCA) or in-stent tissue volume (IVUS).
The relevance of our findings pertains to the widespread use of overlapping DES in major clinical trials. The most critical issue raised by this study relates to the long-term effects of chronic inflammation, including eosinophilic infiltrate, persistent fibrin, and delayed endothelisation in overlapping DES segments. The poor endothelisation may be the result of higher drug dose or may be secondary to hypersensitivity reaction (eosinophilic infiltrate) to the polymer; either or both mechanisms are likely responsible. Because the clinical course of complete endothelisation after placement of overlapping DES is unknown, the duration of any strategies to deal with unanticipated interruption of antiplatelet therapy needs to be further investigated. Nonetheless, patients receiving overlapping DES likely require antiplatelet therapy for a much longer time period than used in major clinical trials.
Although complete healing after single DES placement in animals is seen only after 90 days, in humans the time course of complete healing is unknown but likely to extend beyond one year.
A small study by Shin and his group showed that compared with using the same overlapping DESs, PCI with different overlapping DESs exhibited similar effects on the suppression of neointimal hyperplasia and did not increase the side effects of the DES13.
Early 1-year follow-up for overlapping stents in the SIRIUS trial demonstrated a 5.7% target lesion revascularisation rate for 344 patients compared with 4.5% for no overlapped SES in 714 patients. Degertekin reported a 1-year target vessel revascularisation rate of 6.2% for 96 patients with overlapped SES in their own research registry, although the location of restenosis was not reported.
Study limitations
Our study had several limitations; first of all, the assessment of overlapping DES in an animal model using normal non-atherosclerotic arteries may have underestimated the effects of high doses of drug and polymer load on the arterial vasculature because atherosclerotic tissue may intensify the inflammatory response. Current animal models used in the assessment of DES are limited by their ability to replicate human conditions, although results with the pig coronary model have been generally accepted as representative of human responses, albeit with a different time course of healing.
It is still inconclusive whether these results would be similar to those observed in human atherosclerotic coronary arteries.
In conclusion, our study is first to describe the long-term effects of overlapping the most commonly used DES with Axxess Biolimus™ eluting stent on the arterial wall. We found that the type of the stent does not affect the behaviour of the overlap segment in terms of histomorphometric measures at 28 or 180 days. The paclitaxel-eluting stent and the bare metal, BxVelocity stents were approximately equivalent and the sirolimus-eluting stent was somewhat worse in terms of overall performance. Untill further follow-up data in humans, close and long-term follow-up with continued antiplatelet therapy is advisable for patients receiving overlapping stents.
Acknowledgements
This study was supported by the funds from the Janey Briscoe Center for Cardiovascular Research (MC) and DEVAX Inc., Irvine, CA, USA.

