Abstract
Aims: Although intravascular ultrasound (IVUS) is widely used, there is limited published data on its accuracy in defining plaque characteristics in vivo. Optical coherence tomography (OCT) is a high-resolution imaging technique that takes advantage of the pronounced optical contrast between the components of normal and diseased vessels. The aim of this study was to evaluate the ability of conventional grey-scale IVUS in identifying in vivo coronary plaque characteristics, in particular lipid content as a marker of the vulnerable plaque, when compared to OCT.
Methods and results: In patients undergoing cardiac catheterisation, IVUS and OCT imaging was performed. Detailed qualitative analysis of lipid-rich plaque, calcific plaque, and plaque disruption were performed at corresponding sites using both modalities. A total of 146 matched sites were available for analysis. When compared to OCT, sensitivity of IVUS for identification of lipid pools was low (24.1%) but specificity was high (93.9%). The sensitivity and specificity of IVUS for detection of calcific plaque and plaque disruption were respectively 92.9%; 66.4%, and 66.7%; 96.1%.
Conclusions: Conventional grey-scale IVUS may not be a reliable imaging modality for detection of lipid-rich and hence vulnerable plaques. This has important implications in using conventional grey-scale IVUS to identify the vulnerable plaque.
Introduction
The rupture of a pre-existing vulnerable or high-risk plaque in the coronary artery is a major factor resulting in an acute myocardial infarction (AMI), acute coronary syndrome (ACS) or sudden cardiac death1-5. A thin-cap fibroatheroma (TCFA) is recognised to be a variety of vulnerable plaque and has the following morphologic features: 1) a thin fibrous cap < 65 µm; 2) a large lipid pool; and 3) predominance of macrophages near the fibrous cap3. Positive remodelling is also a feature of vulnerable plaque but this can only be ascertained with serial studies in the presence of an identifiable normal segment. Because of these characteristic features, there is great interest in identifying these TCFA vulnerable plaques in advance of rupture. A variety of non-invasive modalities have attempted to identify these plaques in vivo6. Currently, the limited resolution of the non-invasive modalities precludes their use for this indication.
Among invasive imaging modalities, intravascular ultrasound (IVUS) is currently the most widely employed technique for the assessment of atherosclerotic plaques7. Comparative histological studies have previously shown that an echolucent appearance by IVUS correlated with plaque lipid content8. In contrast, calcified lesions were defined by the presence of echogenic plaques with acoustic shadowing9.
Optical coherence tomography (OCT) is an optical analogue of IVUS that has at least an order of magnitude better resolution10. The OCT characteristics of various plaque components have previously been validated in a histology-controlled study. Using histology as a gold standard, this work demonstrated that OCT has greater than 90% sensitivity and specificity for detecting lipid pools and calcific nodules11.
In this study, we sought to assess the sensitivity and specificity of conventional grey-scale IVUS for the identification of lipid pools and hence potentially vulnerable coronary plaques in patients. Specifically, we wanted to determine if an echolucent area on IVUS correlates with signal-poor regions by OCT, which are consistent with lipid-rich areas. Since the fibrous cap thickness and macrophages are below the known resolution of IVUS, we focused on identification of the lipid pool. In addition, other plaque characteristics such as calcification and intimal dissection were evaluated.
Methods
Study population
Consecutive patients undergoing percutaneous coronary intervention not meeting the exclusion criteria were enrolled. Exclusion criteria were: presence of left-main disease, congestive heart failure, renal impairment with baseline creatinine > 1.8 mg/dL (> 133 µmol/L), extreme tortuousity and calcification of the involved artery, and need for primary or emergency angioplasty. IVUS was performed before OCT in all cases. The study was approved by our institution’s review-board and informed consent was obtained from all patients prior to enrollment.
Image acquisition
The culprit lesion was identified on the basis of a coronary angiogram. In patients with stable angina, a tightest lesion on coronary angiography was selected, whereas a lesion with evidence of plaque rupture with or without local thrombus was chosen as a culprit lesion in patients with ACS and AMI. Other information from electrocardiography, nuclear or echocardiographic stress test, and IVUS evaluation was used to confirm the culprit lesion. The IVUS catheter (3.2 Fr, 30-MHz, Boston Scientific, Natick, MA, USA) was first advanced distal to the culprit lesion and motorised pullback at 0.5 mm/s performed. The technique of intravascular OCT has previously been described10. Briefly, following administration of 100-300 µg of intracoronary nitroglycerin, a 3.2 Fr OCT catheter was advanced through a 7 Fr guiding catheter and over a 0.014-inch coronary guidewire under fluoroscopic guidance to the culprit lesion. Three locations were imaged per lesion: distal and proximal shoulder regions and the area of greatest stenosis or ulceration within the plaque. Following imaging of the centre of the culprit plaque, the catheter was then placed at both proximal and distal shoulder regions for repeat imaging. All imaging locations were documented using contrast angiography. At each location images were acquired while saline (6-10 ml) was introduced through the guide catheter by hand injection to temporarily displace blood. When present, additional lesions within the culprit vessel that exceeded 30% diameter stenosis were imaged in the same fashion. All OCT images were stored digitally for off-line analysis.
Fluoroscopic identification of the IVUS and OCT catheter positions within a vessel was used for localisation of each interrogated site. The coronary guiding catheter tip, nearby side branches and calcification served as fiduciary points. Measurement of time from various landmarks to the regions of interest enabled distance measurement from these reference sites: 0.5 mm x time(s). Using these techniques, the angiographic, OCT, and IVUS image locations of all examined sites could be determined and hence correlated. IVUS images were recorded into S-VHS videotapes for subsequent analysis.
Data collection and analysis
Demographic and clinical data were collected prospectively. At each specified section, OCT images were analysed by two independent investigators (BB and GT) blinded to the clinical presentation using validated criteria for plaque characterisation as previously described11. When there was discordance between observers, a consensus reading was obtained. The corresponding IVUS images at each section were analysed in like manner by two investigators (YK and AL). Because of a 10 fold lower resolution with IVUS, only intraplaque hypoechoic zones with a thickness > 300 µm was defined as an area of low echogenicity (and hence correlate of lipid content). The position of the echolucent zone was defined as “shallow” if it localised in the superficial half of the vessel wall closer to the lumen, and “deep” if the echolucent zone was present within the deeper half of the vessel. On OCT imaging, lipid-rich areas were identified by diffusely bordered, signal-poor regions. For both OCT and IVUS imaging, lipid content was also semi-quantified as the number of involved quadrants on the cross-sectional image. When echolucency occupied two or more quadrants, the plaque was considered lipid-rich12. For IVUS, calcific deposits were identified by highly echogenic segments having a density greater than that of the adventitia and causing acoustic shadowing. In contrast, calcified areas are signal-poor regions with sharply delineated borders on OCT imaging. Intimal dissection was identified by a break in the luminal surface with either modality. Consensus reading was obtained for each section imaged. Inter-observer and intra-observer variabilities were assessed by evaluation of all images by two independent readers and by the same reader after an interval of at least two weeks. The OCT findings of these patients have previously been described12.
Statistical analysis
Comparison of OCT and IVUS classification was made by calculating the sensitivity, specificity, and positive predictive values for each characteristic. Categorical data were analysed by use of the χ2 or Fisher’s exact test as appropriate. Intra-observer and inter-observer variabilities, and concordance of IVUS with OCT were measured by a k test of concordance. A negative value indicates a worse than chance agreement, a value of zero indicates agreement no better than chance, and a value of 1.00 indicates perfect agreement. A κ>0.75 is generally considered to indicate excellent agreement, 0.40<κ<0.75 intermediate to good agreement, while a κ<0.40 indicates poor agreement. Data were compiled in MS Excel 2000 (Microsoft Office 2000, Redmond, WA, USA), and analysed in Intercooled Stata 8.0 (Stata Corp, College Station, TX, USA). A P value ≤0.05 was required for statistical significance. In this study, we have arbitrarily specified OCT as the “gold-standard” for sensitivity and specificity analysis by IVUS. We have also arbitrarily defined sensitivity and specificity as low/poor (<50%), intermediate (50-90%), and high/excellent (>90%).
Results
A total of 69 patients were enrolled in the study. Twelve patients were excluded because imaging was performed after stenting (n=7), the image quality precluded analysis (n=4), or the equipment malfunctioned (n=1). In the remaining 57 patients, 20 patients had a recent ST-elevation AMI, 20 patients had ACS, and 17 patients had SAP. The distribution of the culprit vessels are: left anterior descending artery, 13 patients; left circumflex artery, 12 patients; right coronary artery, 32 patients.
Quantitative assessment of plaque composition
IVUS assessment
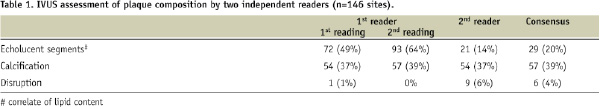
A total of 146 sites had satisfactory OCT and IVUS images for comparative analysis. Echolucent sites consistent with lipid pools were identified in 49% and 64%, respectively of images on repeat readings with IVUS by the same reader. The second reader who had a higher threshold for echolucency, identified only 14% of IVUS images as having echolucent areas. Consensus reading identified echolucent areas in 20% of images. Calcification was reported in 37% and 39%, respectively with IVUS by the first reader and 37% by the second reader. Consensus reading identified calcification in 39%. Intimal dissection was observed in 1% and 0% respectively by the first reader and 6% by the second reader. Consensus reading identified intimal dissection in 4% (Table 1).
OCT assessment
With OCT, areas correlating with lipid content were identified in 77% of corresponding images. Calcification, however, was observed in only 10% by OCT, and intimal dissection was seen in a similar percentage (10%).
Intra-observer and inter-observer reproducibility
Intra-observer agreement for calcification by IVUS was excellent (κ=0.81). For the same observer, there was intermediate to good reproducibility for lipid content but poor reproducibility for intimal dissection (κ=0.56 and κ=0, respectively). When we considered inter-observer reproducibility, the κ statistic fell across all components: Reproducibility for calcification was intermediate to good, but the identification of lipid content was poor (κ=0.63 and κ=0.13, respectively). The intra-observer and inter-observer variability for OCT with these same patients has been previously described12. Intra-observer variability yielded acceptable concordance for lipid-rich plaque (κ=0.86), intimal dissection (κ=0.68), and calcification (κ=0.88). Inter-observer variability was slightly lower: lipid-rich plaque (κ=0.47), intimal dissection (κ=0.66), and calcification (κ=0.57).
Correlation of IVUS findings to OCT
There was overall poor agreement of IVUS and OCT for the various plaque morphologies (Table 2).
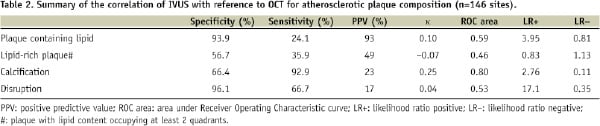
Using OCT as a gold standard, the sensitivity for IVUS detection of lipid content was low (24.1%), but specificity was good (93.9%) (Figures 1 and 2).
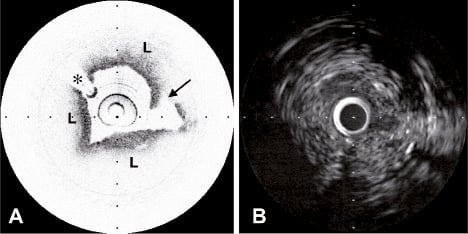
Figure 1. A, Lipid-rich plaque (L) with localised intimal disruption (arrow) that can be clearly seen on OCT. B, Corresponding IVUS image. The intimal disruption is not evident and identification of lipid-rich areas is difficult. * represents guidewire artefact.
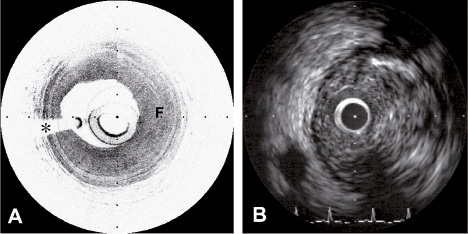
Figure 2. A, Fibrous plaque (F) as seen by OCT. B, The presence of acoustic shadowing within the walls of the plaque make a definitive identification difficult on IVUS. * represents guidewire artefact.
Although detection of calcification had the highest reproducibility, its specificity was only intermediate (66.4%) with excellent sensitivity (92.9%). The pattern for detection of intimal dissection was opposite to that of calcification; there was high specificity (96.1%) but intermediate sensitivity (66.7%).
There was no improvement in IVUS reproducibility when we stratified lipid content into deep or superficial location as well as number of quadrants involved. IVUS consistently under-reported lipid content when compared to OCT (Table 3).
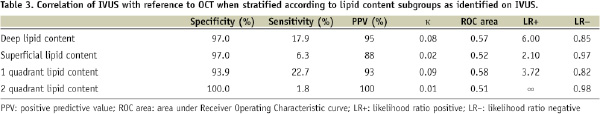
Lipid content occupying two quadrants were identified in only 2 of 146 comparative segments and no segments occupying three or more quadrants were observed with IVUS. In contrast, lipid content occupying 1, 2, 3, and 4 quadrants were seen in 19.3%, 21.4%, 10.3%, and 7.6%, respectively with OCT. Lipid-rich plaques as defined by the presence of lipid content occupying at least two quadrants were therefore observed in 39.3% of sections by OCT in contrast to less than 2% by IVUS (P=0.025). The specificity and sensitivity of IVUS to detect lipid-rich plaques was 56.7% and 35.9% respectively, with poor correlation to OCT (κ= –0.07).
Discussion
This is one of the first in vivo studies correlating conventional grey-scale IVUS findings to OCT in patients with a range of clinical presentations. We have focused on patients with symptomatic CAD with plaque types that are generally more complex and heterogeneous. Previous ex vivo studies have suggested that standard grey-scale IVUS may not be adequate for the detection of vulnerable plaques13,14,26,27. Even with high-resolution 40 MHz IVUS catheters, the identification of lipid pools has a sensitivity of only 65% when compared to histology13. In contrast, the sensitivity of OCT in the detection of lipid rich plaques is above 90%11. Importantly, published in vivo IVUS data have been conflicting. An early IVUS study evaluating the morphological characteristics of coronary plaques in patients with stable versus unstable coronary syndrome showed that positive remodelling and large plaque areas were associated with an unstable clinical presentation while negative remodelling was more common in patients with a stable clinical presentation15. Among patients with unstable angina, echolucent plaques were present in only 19% of the cohort. In contrast, a contemporaneous study documented echolucent plaques in 83% of patients with acute coronary syndromes16. This discrepant IVUS findings have been addressed in a recent review17.
General consensus is that conventional IVUS is inadequate for the detection of vulnerable plaques but there has been few studies comparing it to another imaging modality in the in vivo setting. This study therefore supports current consensus that conventional grey-scale IVUS may not be adequate in identifying the TCFA vulnerable plaque. The low sensitivity relative to OCT obtained in this study may be a result of the lower resolution and tissue contrast provided by IVUS. Recent studies employing a multi-modality approach consisting of coronary angioscopy, IVUS radiofrequency analysis, as well as intravascular magnetic resonance spectroscopy, have confirmed our findings of improved detection of the vulnerable plaque by OCT28,29.
Considering other plaque morphologies, IVUS appears most useful for the detection of intimal dissection (sensitivity of 66.7% and specificity of 96.1%). The detection of blood flow below the intimal flap or within the plaque cavity could explain this good sensitivity. While its sensitivity for detection of calcification is excellent, its specificity is only 66.4%. This is likely consequent to the presence of dense fibrous tissue and associated shadowing which results in misclassification for calcification as was previously described13.
IVUS is an invaluable tool in coronary intervention and is widely used to determine luminal diameter, adequacy of stent expansion, calcification, and importantly, the identification of complications associated with coronary intervention. Specifically, intimal dissection and catheter dissections are important features that can be visualised and our study supports the usefulness of IVUS in this regard. This study does not suggest an inadequacy of conventional IVUS imaging in these settings and certainly does not dissipate its usefulness. Our aim was to determine the concordance of IVUS and OCT in the detection of lipid-rich lesions. Our findings do not support the assertion that conventional grey-scale IVUS in its current iteration is an adequately sensitive tool for the detection of lipid pools, and hence suspected vulnerable plaques. Moreover, the detection of a thin cap (defined as < 65 µm) is difficult since the current best resolution of IVUS is 100 µm. Importantly, the low sensitivity in detection of echolucent segments (and therefore lipid pools) within a plaque can result in misclassification of a TCFA to a stable, fibrous plaque. This may have important implications where the identification of vulnerable plaques could guide emerging therapeutics designed to stabilise the vulnerable plaque. Reliance on conventional grey-scale IVUS may therefore miss a large proportion of TCFA vulnerable plaques.
The imaging of the vulnerable plaque by ultrasound is an important and difficult issue but rapidly evolving techniques have improved this possibility17. Spectral analysis of the backscattered IVUS signals has been proposed to improve in vivo plaque classification18. This has resulted in Virtual Histology IVUS emerging as a promising modality in identifying lipid in plaques. In one study, Virtual Histology IVUS correlated well with histopathologic examination with an 87.1% predictive value for fibrofatty tissue, 88.3% for necrotic core, and 96.5% for dense calcium lesions19. IVUS elastography, which measures vascular wall strain, has also been proposed as an alternative method20. A recent post-mortem analysis documented a high sensitivity and specificity for the detection of regional strain which may identify the vulnerable plaque21. Wavelet analysis is another technique that yielded a high sensitivity for detecting lipid in plaques22. Integrated backscatter IVUS is another evolving methodology that shows promise23. With the exception of Virtual Histology IVUS, however, all of these techniques require further clinical validation and are not yet commercially available. In addition, they all suffer from an inherent limitation of IVUS resolution.
A major limitation of OCT is the need for a blood-free imaging zone. This was achieved through intermittent saline flushes from the guiding catheter. With this flushing technique and the image acquisition speed of current OCT systems, the imaging of long arterial segments was not possible. This is in contrast to IVUS where good penetration through blood allows continuous pullback assessment of the arterial segment. Although great care was taken to ensure precise comparative site assessments, errors in site determination may have resulted in some discrepancy between the two modalities for very short deposits. A further limitation is the relatively shallow axial penetration of OCT (2 mm) in contrast to IVUS which has a depth of up to 8 mm. However, because the most important morphological determinants of plaque vulnerability such as intimal dissection and lipid pools are superficial, the region of greatest interest is within the imaging range of current OCT systems. Other features of plaque vulnerability such as a large plaque volume and positive remodelling therefore cannot be adequately evaluated with the current OCT system. Despite its resolution OCT cannot distinguish necrotic core from lipid pool. Enhancements to OCT technology are anticipated to improve penetration depth and provide significantly faster acquisition rates24,25. This might allow visualisation of long coronary segments using a more rapid pullback mechanism and eliminate many of the technical limitations of the current study. This study was conducted with the use of 30 MHz IVUS catheters which are currently infrequently used. The improved resolution with the current 40MHz catheters (with an expected improvement of resolution from 150 µm to 120 µm) might improve detection of echolucent areas and intimal dissection but we do not expect significant differences from our findings. A small post-mortem study had suggested that OCT may misclassify calcium and lipid rich plaques30. This, however, was not observed in other OCT studies26,27. Importantly, in vivo studies employing other modalities, such as IVUS radiofrequency data analysis and intravascular magnetic resonance spectroscopy, confirmed that OCT could accurately discriminate the majority of lipid rich and calcified plaques28,29.
Although standard IVUS grey-scale imaging is a widely employed technique in the evaluation of atherosclerotic disease, its low correlation with OCT for detection of lipid-rich areas suggest it may not be an adequate tool for the detection of suspected TCFA vulnerable plaques. Emerging technology such as the spectral analysis of backscattered signals, wavelet analysis, and IVUS elastography will likely offset this limitation.
Acknowledgements
We thank our research staff at the Cardiovascular Clinical Research Office, and nurses and technologists at the cardiac catheterisation laboratories of the Massachusetts General Hospital, MA, USA. Funding for work described was provided by the Center for Integration of Medicine and Innovative Technology, the National Institutes of Health and through a generous gift from Mr. and Mrs. John and Marilee Polmonari. AF Low is the recipient of a Health Manpower Development Program Fellowship funded by the Ministry of Health, Singapore as well as a National Medical Research Council Fellowship, Singapore.

