Abstract
Calcified nodules (CNs) represent significant challenges in percutaneous coronary intervention (PCI) due to their complex morphology, variable treatment responses, and association with stent failure due to reprotrusion or stent underexpansion. CNs are classified into eruptive and non-eruptive subtypes, each with distinct histological features and prognostic implications. Eruptive CNs are biologically active, being associated with a disrupted fibrous cap, overlying thrombus, and intraplaque haemorrhage, and they are thus more readily deformable by balloon dilation during PCI. Non-eruptive CNs, or nodular calcifications, tend to be stable, with an intact fibrous cap, and their deformability varies depending on the composition and base of the nodules. Enhanced angiography and intravascular imaging have greatly improved our understanding of CNs and may help to accurately identify nodule subtypes and guide treatment. Furthermore, understanding the deformability of CNs is crucial for optimising treatment outcomes. In this review, we discuss the identification and management of CNs in the context of PCI.
A calcified nodule (CN) is a distinct type of calcified coronary lesion that protrudes into the lumen with a convex luminal surface and is often accompanied by adjacent segments with heavily calcified sheets1. Due to its unique pathogenesis, a CN is frequently found at hinge points within the coronary artery or in larger parts of the artery with a high lipid burden and propensity for a necrotic core23. The presence of severe angiographic calcification or a radiolucent mass may raise suspicion for CNs. Although CNs have a typical angiographic appearance, diagnosing CNs based solely on angiography can be challenging, as they may still be present without distinct angiographic features4. The use of intravascular imaging, such as optical coherence tomography (OCT) and high-definition (HD) intravascular ultrasound (IVUS), has significantly advanced the recognition and understanding of CNs1. In addition, the use of enhanced angiography may provide critical details of the CN subtype.
CNs can be categorised into two distinct subtypes based on their predominant morphological features: (1) eruptive CNs, which are active in nature and can cause acute coronary syndrome (ACS); and (2) non-eruptive CNs or nodular calcification, which are more stable and typically present as chronic coronary syndromes or remain asymptomatic. In histological studies, the term “CNs” generally refers to eruptive CNs, which are associated with culprit lesions for ACS or sudden cardiac death, whereas non-eruptive CNs are classified as nodular calcification.
CNs are among the most challenging lesion subsets to treat with percutaneous coronary intervention (PCI), carrying an increased risk of adverse outcomes due to stent underexpansion caused by non-deformed CNs behind the stent or reprotrusion of the CNs through the stent struts56789101112. Although the two CN subtypes can coexist and may represent different stages of the disease process, understanding their natural histories, differential responses to specific treatments, and prognoses after stent implantation, as well as identifying them through enhanced angiography and intravascular imaging may have important clinical implications1. In this review, we discuss the identification and classification of CNs, their characteristics, and strategies for effective treatment.
Epidemiology
Autopsy studies have shown that plaque rupture and erosion are the most common causes of acute coronary thrombosis leading to sudden cardiac death, while eruptive CNs are the least frequent cause31314. Similar patterns have been observed in clinical studies of ACS patients. In the Massachusetts General Hospital OCT Registry, which included 126 ACS patients who underwent pre-PCI OCT imaging, the incidences of plaque rupture, plaque erosion, and CNs were 43.7%, 31.0%, and 7.9%, respectively15. More recently, the Tokyo, Kanagawa, Chiba, Shizuoka, and Ibaraki active OCT applications for ACS (TACTICS) registry also demonstrated CNs as the least common cause of ACS (4.0%), compared with plaque rupture (59.1%) and erosion (25.6%)16. These findings are consistent with other studies, which have reported a prevalence of CNs ranging from 2% to 8% in ACS patients2101517.
Nevertheless, recent studies indicate that CNs are more prevalent in routine clinical practice than previously expected. In a Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) substudy, which utilised 3-vessel IVUS to assess non-culprit lesions, CNs were identified in 17% of analysable arteries and 30% of patients18. CNs have been found to be particularly prevalent in calcified lesions; approximately one-third of severely calcified culprit lesions in ACS patients were attributed to CNs in one study2. Furthermore, intravascular imaging has identified CNs in up to 50% of calcified lesions treated with rotational atherectomy (RA)9. These findings highlight the need for operators to consider the potential presence of CNs when encountering heavily calcified coronary lesions.
Studies have also found that the prevalence of CNs is higher in patients with diabetes mellitus, prior bypass graft surgery, or those undergoing haemodialysis2. Notably, CNs were commonly found in approximately 40% of culprit lesions in ACS patients with chronic kidney disease1516. A recent longitudinal study investigating the natural history of CNs using serial OCT has confirmed that the development of de novo CNs is strongly associated with haemodialysis19. Additionally, a larger calcium burden with residual lipidic plaque, left main bifurcation location, and a greater in-lesion angiographic Δ angle – reflecting increased hinge motion, as seen in the mid-right coronary artery – were associated with CN development, providing important insights into its pathophysiology19.
Pathophysiology
Although the exact pathogenesis of CNs remains unclear, pathological and clinical findings offer valuable insights. An autopsy study of patients who experienced sudden cardiac death identified several key histopathological features of CNs3.
Histologically, eruptive CNs were characterised by multiple nodular fragments of dense calcification penetrating the overlying fibrous cap, accompanied by endothelial cell loss, and protruding into the luminal space with an overlying fibrin/platelet thrombus (Figure 1)3. Notably, necrotic core calcifications, which lack collagen, were predominantly found in the culprit segments of CNs, whereas collagen-rich calcifications were identified in the proximal and distal flanking segments (Figure 1)3. These morphological features suggest that an eruptive CN may arise from the breakdown of collagen-deficient necrotic core calcifications, which are sandwiched by the rigid, collagen-rich calcified sheets3. Alternatively, the movement of the artery may prevent collagenisation of a calcified sheet at the hinge, leading to the formation of calcium fragments that are unable to join but prone to eruption due to artery movement. Regardless, mechanical forces due to coronary hinge motion are believed to play a critical role in the evolution of eruptive CNs, as collagen-deficient necrotic core calcifications are more susceptible to mechanical stress compared with the flanking collagen-rich calcified sheets (Figure 2). Their predilection for the mid-right coronary artery is consistent with this hinge theory as it is an area of high torsion. However, it should be noted that this may not be the only mechanistic explanation, as CNs can also be observed in arteries without significant hinge motion.
Angiogenesis within CNs may also contribute to their evolution, as fibrin deposits of varying ages are frequently observed, suggesting repeated episodes of capillary damage or leakage (Figure 1). This cyclical process of capillary disruption and fibrin deposition may drive the progressive growth and extrusion of nodular calcifications, eventually leading to their protrusion into the lumen. Fragmented, small calcified nodules can disrupt the overlying fibrous cap and endothelial lining, triggering platelet aggregation and fibrin deposition, which promote luminal thrombus formation. Intraplaque haemorrhage from capillary disruption and the development of an overlying thrombus may ultimately contribute to acute luminal narrowing and the onset of ACS13.
Although the mechanisms of development of non-eruptive CNs, or nodular calcification, remain unknown, one of the hypotheses is that a non-eruptive CN may be a healed form of an eruptive CN, sharing a similar underlying mechanism312. The intraplaque haemorrhage within an eruptive CN may resolve through osteogenic transformation of blood components, which integrates small calcified fragments into a larger calcified mass, accompanied by the healing of the disrupted fibrous cap1320. This is supported by histological findings, which frequently reveal fibrin between calcified spicules, along with osteoclasts and inflammatory cells14. In addition, calcification may progress into the necrotic core and residual lipidic plaque, gradually reducing their volume as the CN matures31921. Consequently, it is hypothesised that non-eruptive CNs may be associated with predominantly soft (lipid/fibrin) (Supplementary Figure 1A) or hard (calcific) (Supplementary Figure 1B) components, which could have clinical implications for differing treatment responses, particularly when distinguishing between deformable and non-deformable CNs122.
A recent clinical study by Sugizaki et al further supports these hypotheses19. In this natural history study, untreated calcified lesions were followed with repeat OCT, and the development of de novo CNs was associated with the presence of attenuated calcium, suggesting a residual lipidic component19. This finding aligns with histological evidence indicating that CNs are more likely to originate from necrotic core calcifications rather than collagen-rich calcifications. Additionally, a larger angiographic Δ angle between systole and diastole was associated with the development of de novo CNs19, reinforcing the role of mechanical forces due to hinge motion in their development.
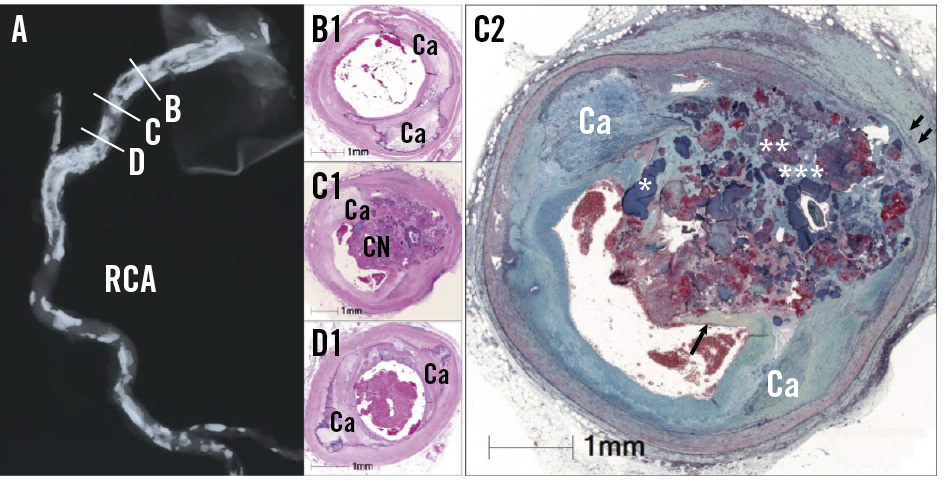
Figure 1. Histology of eruptive CNs. Histology figures reproduced with permission from3. A) Radiograph of the RCA showing extensive calcification. B-D) Cross-sections stained with haematoxylin and eosin showing (B1) flanking sheet calcium (Ca) proximal to (C1) an eruptive calcified nodule at a typical hinge point on the radiograph (C) and (D1) distal flanking sheet calcium. C2) Movat’s pentachromic staining of an eruptive CN showing sheet calcium flanking an eruptive nodule containing dense calcium (*), fibrin with potential calcifying lipid (**), and a mucopolysaccharide matrix (***). The fibrous cap shoulder (arrow) is seen adjacent to adherent thrombus on top of the eruption. The hinge movement has also led to disruption of the vascular media (double arrow). CN: calcified nodule; RCA: right coronary artery
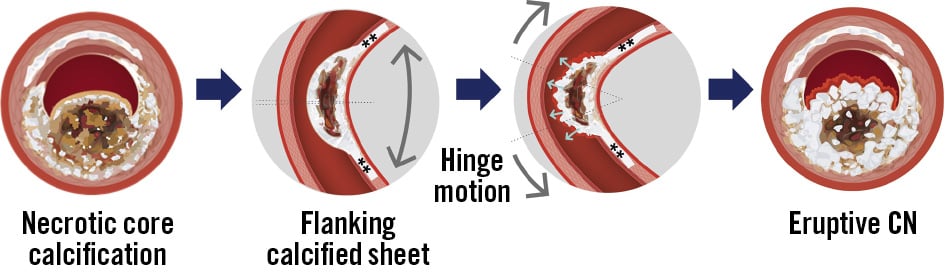
Figure 2. Hinge motion and an eruptive CN. An eruptive CN originates from necrotic core calcification, which lacks collagen and is flanked by segments of sheet calcium (**). Fragmented calcium induces capillary damage, leading to intraplaque haemorrhage and fibrin deposition, which drive its progressive growth. Ultimately, fragmented calcium disrupts the overlying fibrous cap, promoting intraluminal thrombus formation. Grey and white indicate calcium fragments, brown represents necrotic core or lipidic components, and red denotes areas of haemorrhage and fibrin. CN: calcified nodule
Identification and evaluation of CNs
Identification of CNs during PCI is important, since they are associated with increased risks of suboptimal procedural results and adverse clinical events56789101112. The presence of angiographically severe calcification, particularly at the hinge points or the left main bifurcation, should raise suspicion for CNs. Clinical characteristics can also provide important clues: for example, CNs were identified in approximately 40% of culprit lesions in ACS patients with chronic kidney disease or those undergoing haemodialysis1516.
On angiography, a radiolucent filling defect in the lumen during contrast injection (angiographic radiolucent mass [ARM]) is often considered to be a CN (Figure 3)4. However, an ARM can also represent a thrombus, particularly in acute presentations, though severe angiographic calcification increases the likelihood of CN presence. Identifying CNs based on angiography is further complicated by the fact that the absence of an ARM does not rule out the presence of intravascular imaging-defined CNs, which can be present even in the absence of angiographic calcification4. It is also important to note that stenosis severity is often overestimated by angiography in the presence of an ARM4. Enhanced fluoroscopy technology, such as StentBoost (Philips), SyncVision Device Detection (Philips), ClearStent (Siemens Healthineers), StentViz (GE HealthCare), and 3DStent (GE Healthcare), can improve the sensitivity for detecting CNs and is particularly useful for evaluating treatment responses to balloon angioplasty. Nevertheless, angiography alone is still limited in providing a detailed evaluation of CN morphology.
In this regard, intravascular imaging is valuable for diagnosing a CN and distinguishing its predominant subtypes1. Non-HD-IVUS can detect CNs as luminal protrusions of hyperechoic (bright) plaque with significant acoustic shadowing (dark); however, its resolution is often insufficient for further characterisation of CNs. In contrast, HD-IVUS can more reliably distinguish between eruptive and non-eruptive CNs by assessing the continuity of the fibrous cap. Eruptive CNs are characterised by a disrupted fibrous cap, often accompanied by an overlying thrombus, resulting in an irregular surface (Figure 3A1). In contrast, non-eruptive CNs have an intact fibrous cap without an overlying thrombus1.
Compared with IVUS, OCT offers superior spatial resolution, providing additional benefits in evaluating CNs. In OCT, CNs appear as signal-poor, heterogeneous areas protruding into the lumen. An irregular surface, often accompanied by an overlying thrombus, reflects a disrupted fibrous cap, characteristic of an eruptive CN (Supplementary Figure 2A), whereas a smooth, bright surface suggests an intact fibrous cap, indicative of a non-eruptive CN (Supplementary Figure 2B)11220. Unlike collagenous calcified sheets, which have well-demarcated borders, both eruptive and non-eruptive CNs exhibit signal attenuation due to OCT’s limited penetration depth and the presence of necrotic core calcification, intraplaque haemorrhage, and/or residual lipidic component, reflecting their developmental origins. This signal attenuation complicates the assessment of underlying plaque characteristics and components (e.g., lipid or calcium), making it challenging to predict the deformability of CNs, particularly non-eruptive CNs.
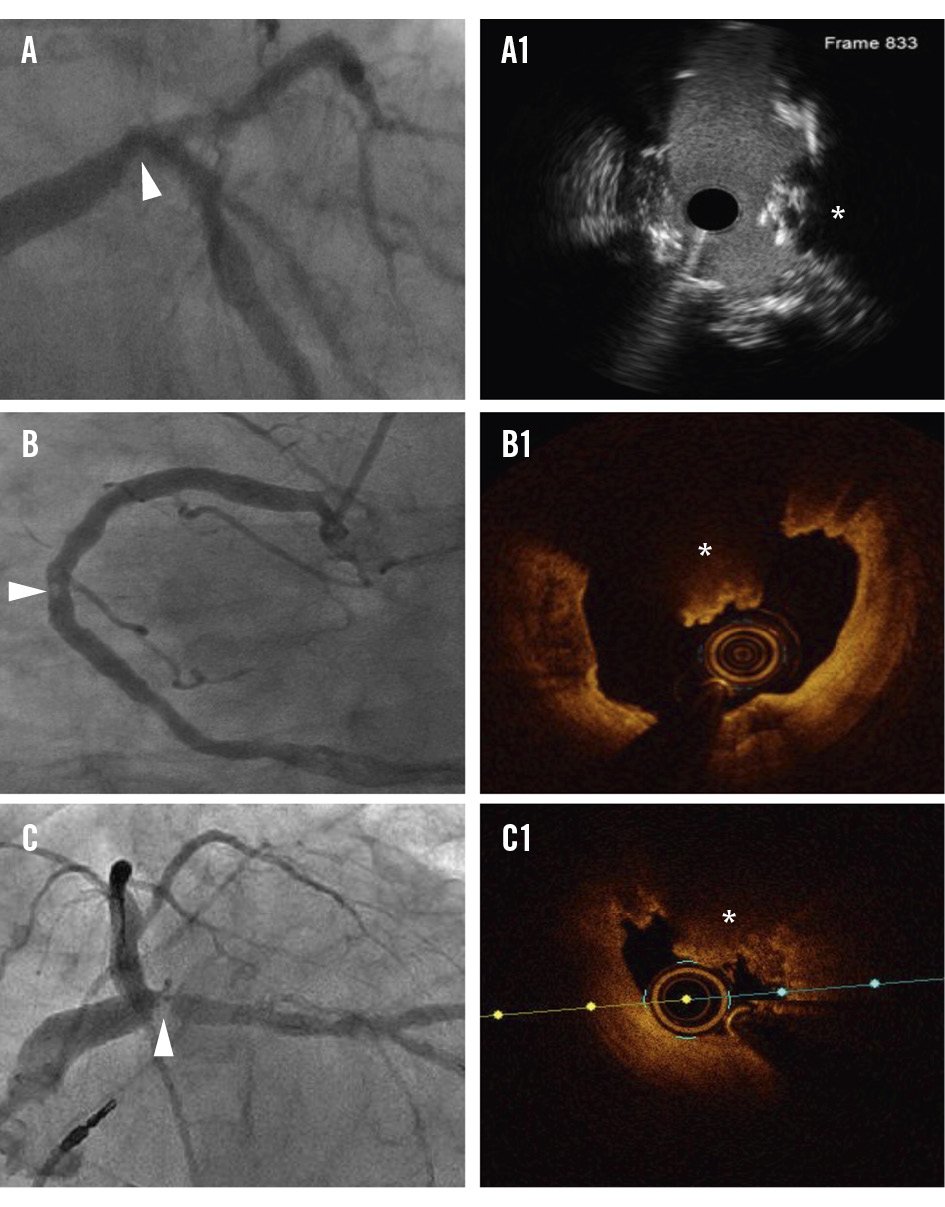
Figure 3. Identification of CNs on angiography. On angiography (A-C), CNs appear as angiographic radiolucent masses (arrowhead). CNs (*) are confirmed using high-definition intravascular ultrasound (A1) or optical coherence tomography (B1, C1). CN: calcified nodule
Treatment challenges and pathobiology
Patients with CNs tend to have worse outcomes after PCI compared with those without CNs56789. Since angiography alone often overestimates stenosis severity in lesions with CNs4, intravascular imaging or physiological assessment may be helpful in accurately determining the need for PCI1. When treating CNs with PCI, several factors contributing to outcomes should be considered: (1) the risk of stent underexpansion due to non-deformable CNs1222; (2) distinct immediate treatment responses between eruptive and non-eruptive CNs and their paradoxical long-term outcomes122022; (3) the risk of early CN reprotrusion through stent struts, potentially leading to in-stent restenosis2324; (4) the risk of stent fracture caused by hinge motion, given the frequent anatomical location of CNs; (5) the presence of heavily calcified sheets adjacent to CNs, often resulting in a minimal stent area (MSA) outside the site of the CN25; and (6) patient-level cardiovascular risk factors commonly associated with CNs, such as chronic kidney disease.
When evaluating the immediate treatment response for PCI, it is important to understand the deformability of CNs (Figure 4, Figure 5). In OCT studies, a CN is considered deformable if there is a visible reduction in lumen protrusion after treatment, accompanied by more symmetric stent expansion, as indicated by an asymmetry index and an eccentricity index greater than 0.72225. Studies have shown that eruptive CNs are almost universally deformable, consistent with their composition of fragmented necrotic core calcification (Figure 4A, Figure 5A). In contrast, while approximately two-thirds of non-eruptive CNs are deformable, the remaining one-third are non-deformable2225. It is hypothesised that non-eruptive CNs associated with underlying calcified components are likely to be non-deformable, as they cannot be compressed against the rigid calcified structure (Figure 4B, Figure 5B). Conversely, those associated with predominantly soft residual lipid are more likely to be deformable, as they can be compressed into the softer central core (Figure 4C, Figure 5C)1. Consequently, greater acute stent expansion can be achieved in eruptive CNs compared with non-eruptive CNs12.
Counterintuitively, clinical outcomes do not always correlate with acute procedural results. Despite their universal deformability and greater acute stent expansion, eruptive CNs have demonstrated worse clinical outcomes after PCI compared with non-eruptive CNs12. Specifically, the target lesion revascularisation (TLR) rate after PCI was significantly higher in eruptive CNs than in non-eruptive CNs (18.3% vs 9.6%; p=0.04)12. The paradoxically worse clinical outcomes of eruptive CNs are believed to result from their more frequent presentation with ACS and the reprotrusion of eruptive nodular material through stent struts, leading to the development of an in-stent CN12324. Notably, the irregular protrusion of CNs within the implanted stent on post-PCI OCT was independently associated with TLR26. Furthermore, TLR predominantly occurred in lesions with a maximum eruptive CN arc >180°, despite acceptable stent expansion27, suggesting that a higher CN burden may also contribute to adverse clinical outcomes after PCI. Additionally, mechanical forces at the hinge site may not only facilitate the reprotrusion by expelling nodular material through the stent struts but also increase the risk of stent fracture, both of which contribute to stent failure1.
Another important consideration in the treatment of CNs is their frequent association with severe calcifications and adjacent calcified sheets with a heavy calcium burden3. In a pooled analysis of the Disrupt CAD studies, the pre-PCI minimal lumen area was colocated with the site of maximum CNs in only 51.7% of cases25. Additionally, the post-PCI MSA was located outside the maximum CN site in 57.7% of cases25. Therefore, treatment strategies should address not only the CNs but also the entire calcified lesion.
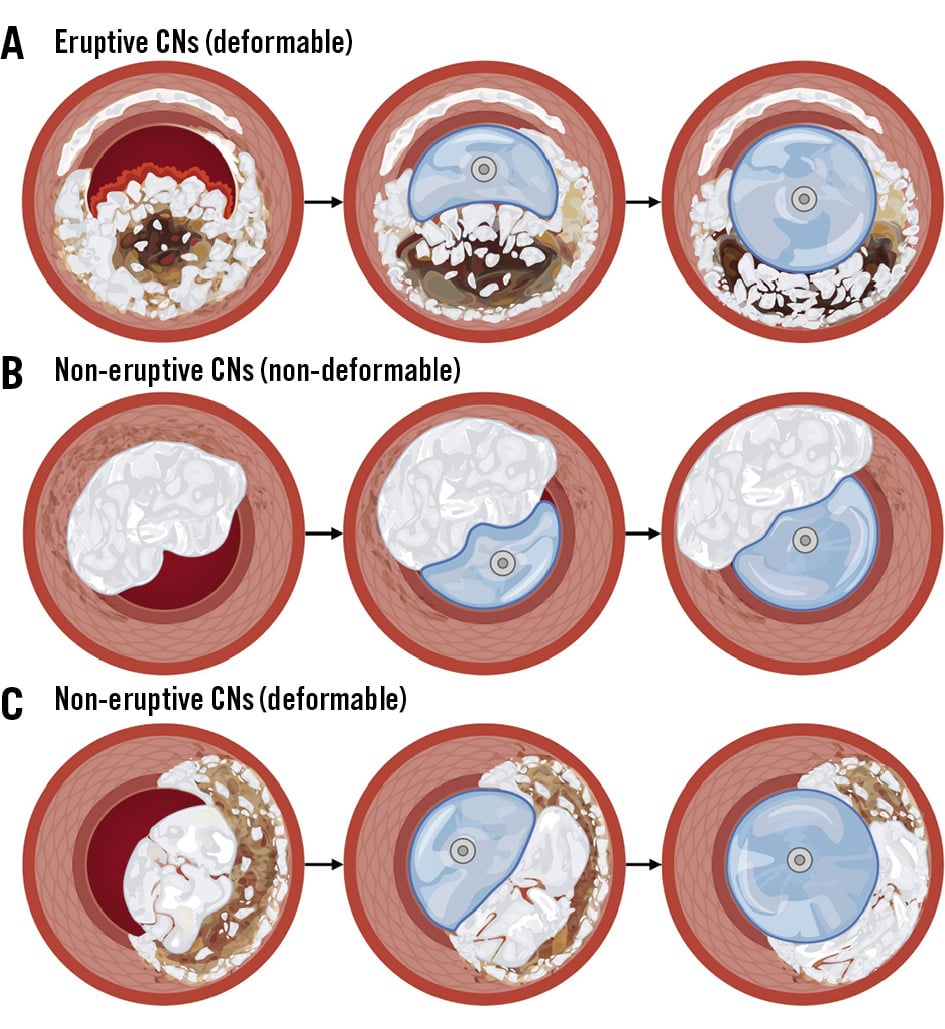
Figure 4. Fundamental concept of deformability of CNs. Illustration of the deformability concept in CNs. A) Eruptive CNs are universally deformable, whereas non-eruptive CNs may be either non-deformable (B) or deformable (C), depending on their composition and the underlying plaque type. CN: calcified nodule
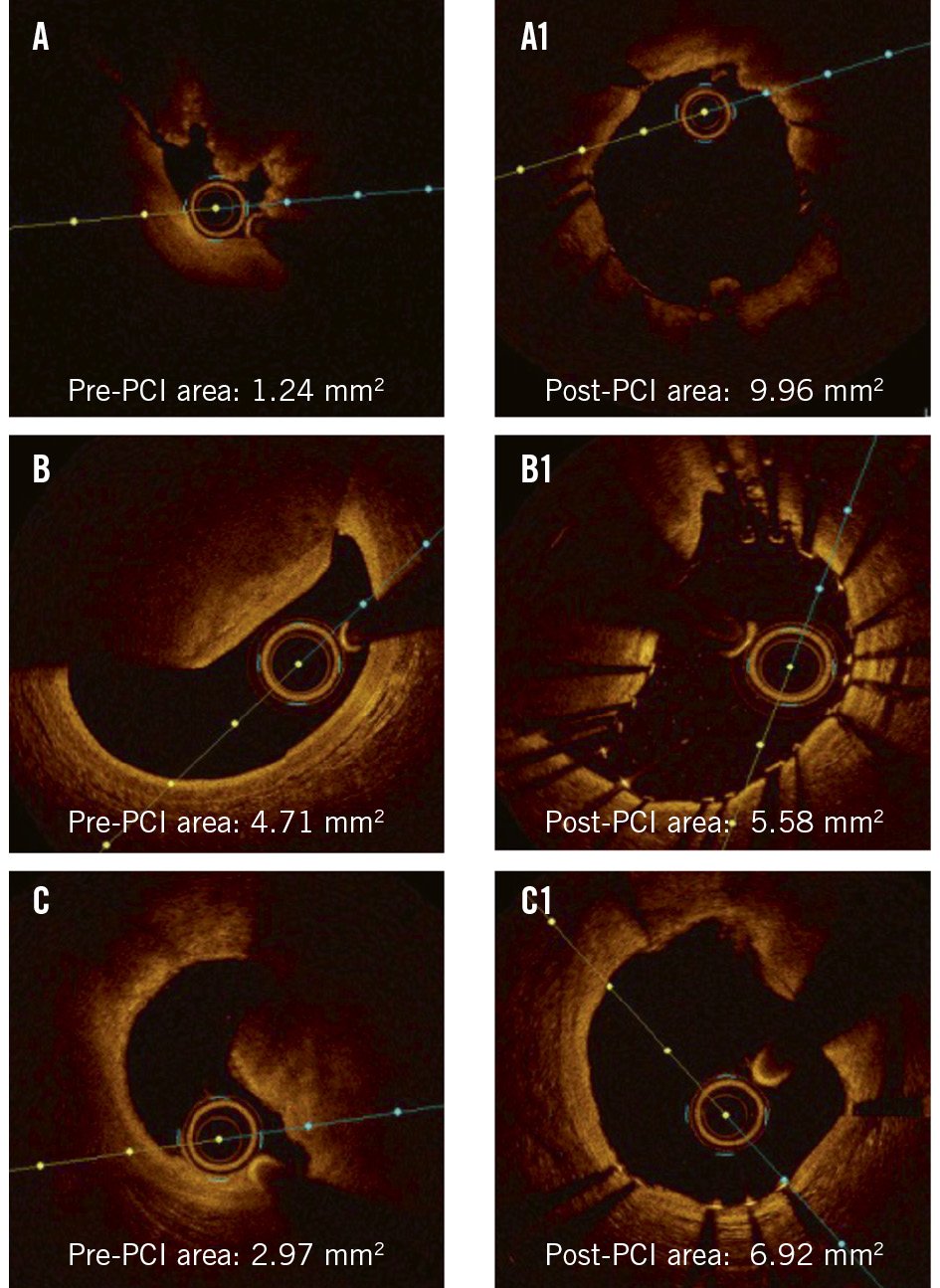
Figure 5. Case examples of deformability of CNs. Case examples demonstrating (A, A1) an eruptive CN, (B, B1) a non-deformable, non-eruptive CN with eccentric stent expansion, and (C, C1) a deformable, non-eruptive CN, before and after PCI. CN: calcified nodule ; PCI: percutaneous coronary intervention
Treatment modalities
Several modalities can be used to modify CNs prior to stent implantation, including non-compliant (NC) or speciality balloon angioplasty, RA or orbital atherectomy (OA), or intravascular lithotripsy (IVL).
Balloon angioplasty
NC balloon angioplasty is the most commonly used lesion preparation technique for treating CNs in routine clinical practice12. Speciality balloons, such as cutting or scoring balloons, may offer theoretical advantages by enabling controlled dilation and reducing balloon slippage, particularly in cases of eccentric CN protrusions1. However, a balloon-only strategy may be limited by eccentric balloon expansion towards the non-CN side of the vessel and insufficient force to effectively modify CNs surrounded by severe calcification1. Furthermore, eccentric balloon expansion can cause dissection at the junction between calcified and non-calcified regions. Despite these limitations, balloon angioplasty, especially when combined with advanced fluoroscopic technologies, can provide valuable insights into the deformability of CNs, which cannot be predicted by intravascular imaging (Supplementary Figure 3). If high-pressure balloon angioplasty using 1:1-sized NC or speciality balloons achieves full balloon expansion in two orthogonal views, it can be assumed that the CN is deformable and may not require additional lesion preparation before stent implantation (Supplementary Figure 3B’)1.
Atherectomy
Atherectomy techniques are used to "modify" heavily calcified coronary plaques and CNs. When utilising atherectomy for CN treatment, it is important to recognise that their efficacy depends on guidewire bias and the size of the device relative to the lumen.
RA is prone to guidewire bias, which can divert the centrally mounted burr away from eccentric CN protrusions, making it difficult to achieve adequate contact and debulking (Figure 6), especially when the lumen is relatively larger than the burr size. Intravascular imaging can aid in predicting guidewire bias by assessing the position of the guidewire relative to the CN location. Using a larger burr may partially compensate for this unfavourable bias. The operator may also consider repositioning the guidewire by withdrawing or advancing it, selecting a stiffer or floppier guidewire, or using a guide extension catheter to change guidewire bias and improve the effectiveness of atherectomy. Nevertheless, RA-assisted PCI has not been shown to reduce the risk of target vessel revascularisation in patients with CNs28.
Compared with RA, OA is theoretically less susceptible to guidewire bias because of its eccentrically mounted crown and circumferential shaving mechanism1, although studies have shown that the crown’s maximum orbital diameter is approximately 1.8 mm29. We have recently shown that the debulking efficacy of OA depends on lumen size (Figure 7)30. In cases where the baseline lumen area was ≤2.5 mm² on OCT, OA achieved a 38.8±18.2% relative reduction in CN size and a 32.9% (interquartile range [IQR] 15.8-52.1) relative lumen gain (Figure 7A). Conversely, when the baseline lumen area was >2.5 mm² at the site of maximum CNs, the debulking effect was significantly less (16.8±11.6%), with only minimal lumen gain (9.2% [IQR 5.3-14.9]) (Figure 7B). In this regard, intravascular imaging may help predict the potential debulking effect of OA and identify patients who are most likely to benefit from this treatment. Additionally, meticulous techniques, including slow and multiple passes of the OA crown across the CN, may enhance its effectiveness, with high-speed OA further increasing debulking. Nevertheless, it should be noted that clinical outcome data regarding the efficacy of OA for CN treatment remain limited.
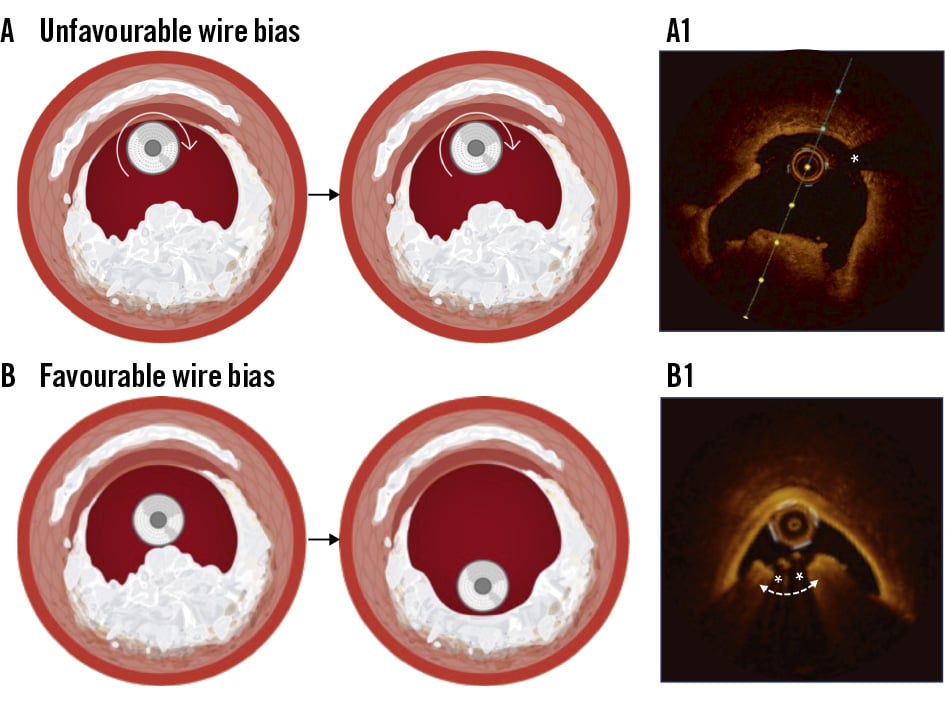
Figure 6. Guidewire bias and atherectomy. A) Unfavourable guidewire bias away from the CN results in minimal debulking. A1) On OCT, the guidewire (*) is positioned on the opposite side of the CN, leading to modification of the opposing calcified plaque but with minimal effect on the CN itself. B) Favourable guidewire positioning (*) adjacent to the CN facilitates effective debulking. B1) On OCT, this optimal guidewire bias results in CN debulking with a groove formation. CN: calcified nodule; OCT: optical coherence tomography
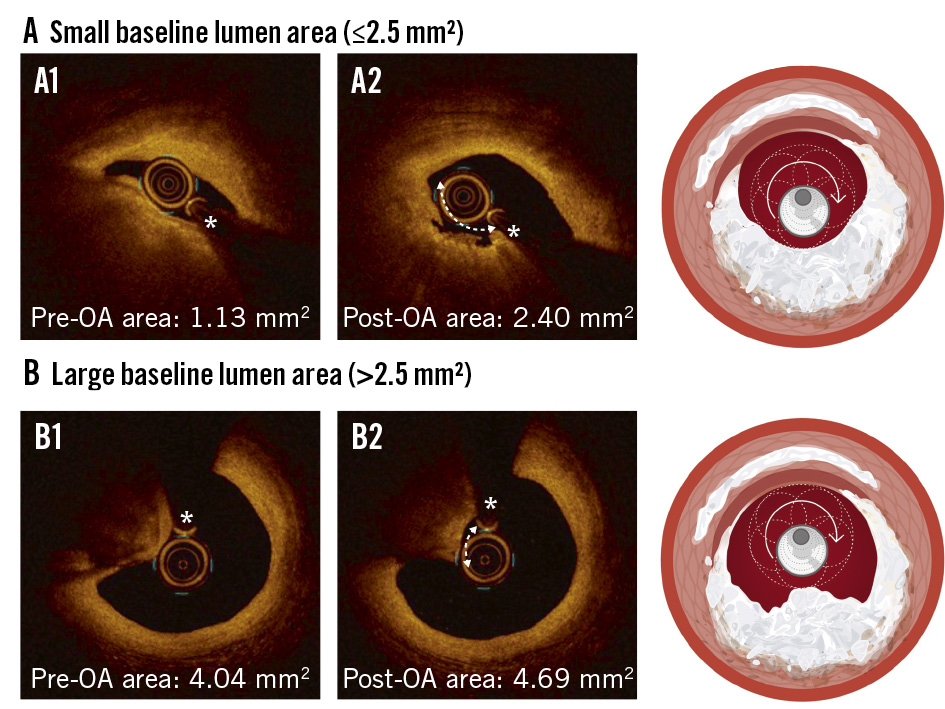
Figure 7. Effect of OA on CNs according to baseline lumen area. A) In lesions with a small baseline lumen area (≤2.5 mm²; A1), OA achieved effective debulking of CNs with significant lumen gain (A2). B) In contrast, OA in lesions with a larger baseline lumen area (>2.5 mm²; B1) resulted in minimal debulking and limited lumen gain (B2). The asterisk denotes the guidewire position. CN: calcified nodule; OA: orbital atherectomy
Intravascular lithotripsy
IVL is increasingly utilised for the treatment of severely calcified coronary lesions because of its ease of use, effectiveness, and favourable safety profile3132. The primary mechanism of IVL is calcium fracture (Figure 8, Supplementary Figure 4), which is not influenced by guidewire bias or the relative size of the device and lumen, as seen with atherectomy devices3334. Instead, its effectiveness is proportional to the calcium burden3334. The unbiased mechanism of IVL is particularly advantageous for preparing the entire calcified lesion, rather than targeting only the CN itself, especially given the presence of rigid calcified sheets with a heavy calcium burden flanking the CNs125.
A recent Disrupt CAD substudy demonstrated the feasibility of IVL for treating severely calcified lesions with CNs, with acceptable procedural and long-term outcomes, comparable to those without CNs25. However, approximately one-third of non-eruptive CNs remained non-deformable despite IVL treatment22, potentially resulting in eccentric stent expansion. Whether this translates into worse clinical outcomes and which advanced lesion preparation strategy is most effective remain unclear. In theory, a dual-preparation strategy combining atherectomy and IVL may be beneficial as atherectomy can ablate portions of the CN, thereby making it more amenable to fracture by IVL. Future studies are warranted to evaluate the clinical utility and potential benefits of this dual-preparation strategy.
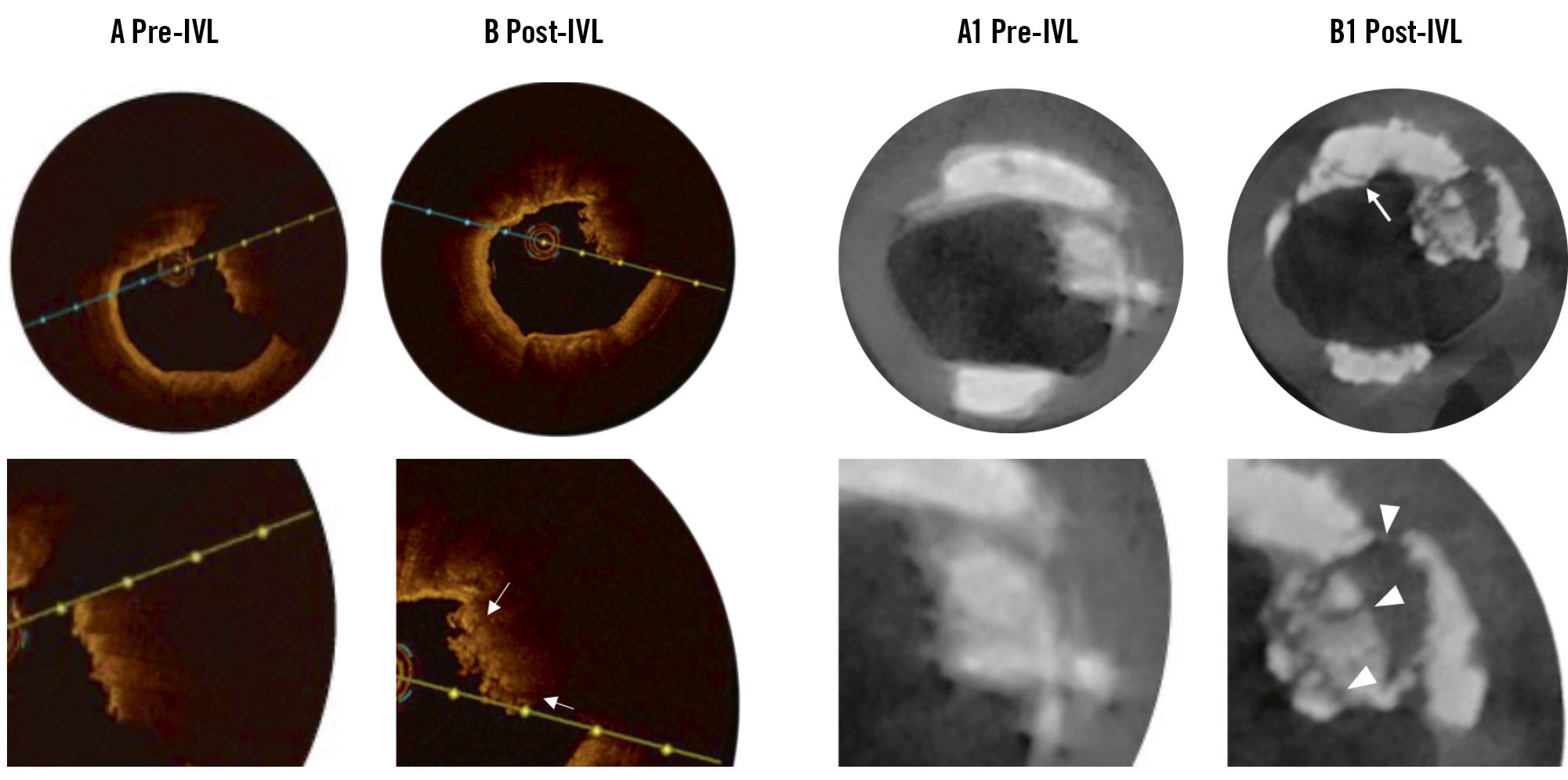
Figure 8. Micro-CT imaging of CNs after IVL treatment. A, A1) Pre-IVL and (B, B1) post-IVL OCT and micro-CT images. After IVL, OCT revealed a few fracture lines (arrows; lower image in B). In micro-CT, multiple fractures are more evident within the CN (arrowheads; lower image in B1) and the calcified sheet (arrow; upper image in B1). CN: calcified nodule; CT: computed tomography; IVL: intravascular lithotripsy; OCT: optical coherence tomography
Proposed treatment approach
We have proposed a potential treatment algorithm for CNs that incorporates intravascular imaging and deformability assessment using balloon angioplasty1. Given the growing evidence supporting the benefits of intravascular imaging-guided PCI in complex coronary lesions35, including those with severe calcifications36, the use of intravascular imaging should be considered, particularly in cases with moderate to severe calcifications detected on angiography.
For both eruptive and non-eruptive CNs, assessing deformability using high-pressure balloon angioplasty with 1:1-sized NC or speciality balloons is a reasonable and practical initial approach. Eruptive CNs are more likely to be deformable, whereas non-eruptive CNs may be non-deformable. If full balloon expansion is not achieved in two orthogonal views using enhanced fluoroscopy, the CNs are likely non-deformable and may require advanced lesion preparation strategies. The choice of strategy should be guided by additional factors, including operator preference, lumen size at the CN site, and the presence of guidewire bias, as discussed above. In cases where device delivery is not possible or when the lesion severity and burden of calcification are high, as indicated by a calcium scoring system37, upfront advanced lesion preparation can be considered.
Regardless of the modality used, achieving full balloon expansion on angiography is ideal before stent implantation. While IVL has been reported as a treatment for stent underexpansion38, its use in cases of acute underexpansion of a freshly implanted stent may pose a risk of polymer damage, although an in vitro study has shown that the extent of such damage is minimal39.
Future directions
Until now, the deformability of CNs could only be determined by assessing balloon expansion, largely because intravascular imaging is unable to visualise entire CNs and underlying plaque characteristics, which, in part, determine the deformability of CNs1. With emerging data supporting the use of coronary computed tomography angiography (CCTA) in PCI, evaluation of CN morphology, density, and associated plaques via CCTA may provide further characterisation of a CN and help predict its deformability in advance. In addition, the use of IVUS-near-infrared spectroscopy (NIRS) or OCT-NIRS may help to evaluate the presence of residual lipidic plaque associated with CNs.
Another potential approach is the use of a drug-coated balloon (DCB). DCBs may be theoretically better for the treatment of CNs than drug-eluting stents40, particularly given the mechanisms of stent failure, such as stent fracture due to hinge motion and the reprotrusion of CNs through stent struts, which can lead to the development of in-stent CNs1. However, clinical data on this strategy are currently lacking, and its theoretical benefits must be weighed against the risks of recoil and regrowth of CNs, which can be substantial140. Furthermore, DCBs can only be considered when lesions with CNs are adequately modified and prepared, reinforcing the importance of proper evaluation and treatment of CNs.
Conclusions
A CN is a distinct type of calcified coronary lesion and represents one of the most challenging subsets to treat with PCI. Enhanced angiography and intravascular imaging offer the ability to identify CNs and distinguish between their subtypes − eruptive and non-eruptive − which demonstrate distinct morphological features, treatment responses, and long-term prognoses after PCI (Central illustration). A thorough understanding of these characteristics, along with current evidence, is essential to guide operators in selecting the most effective treatment strategies. However, despite the use of advanced lesion preparation techniques, outcomes after PCI for CNs remain suboptimal. Therefore, further efforts are needed to enhance and refine treatment approaches to improve clinical outcomes.
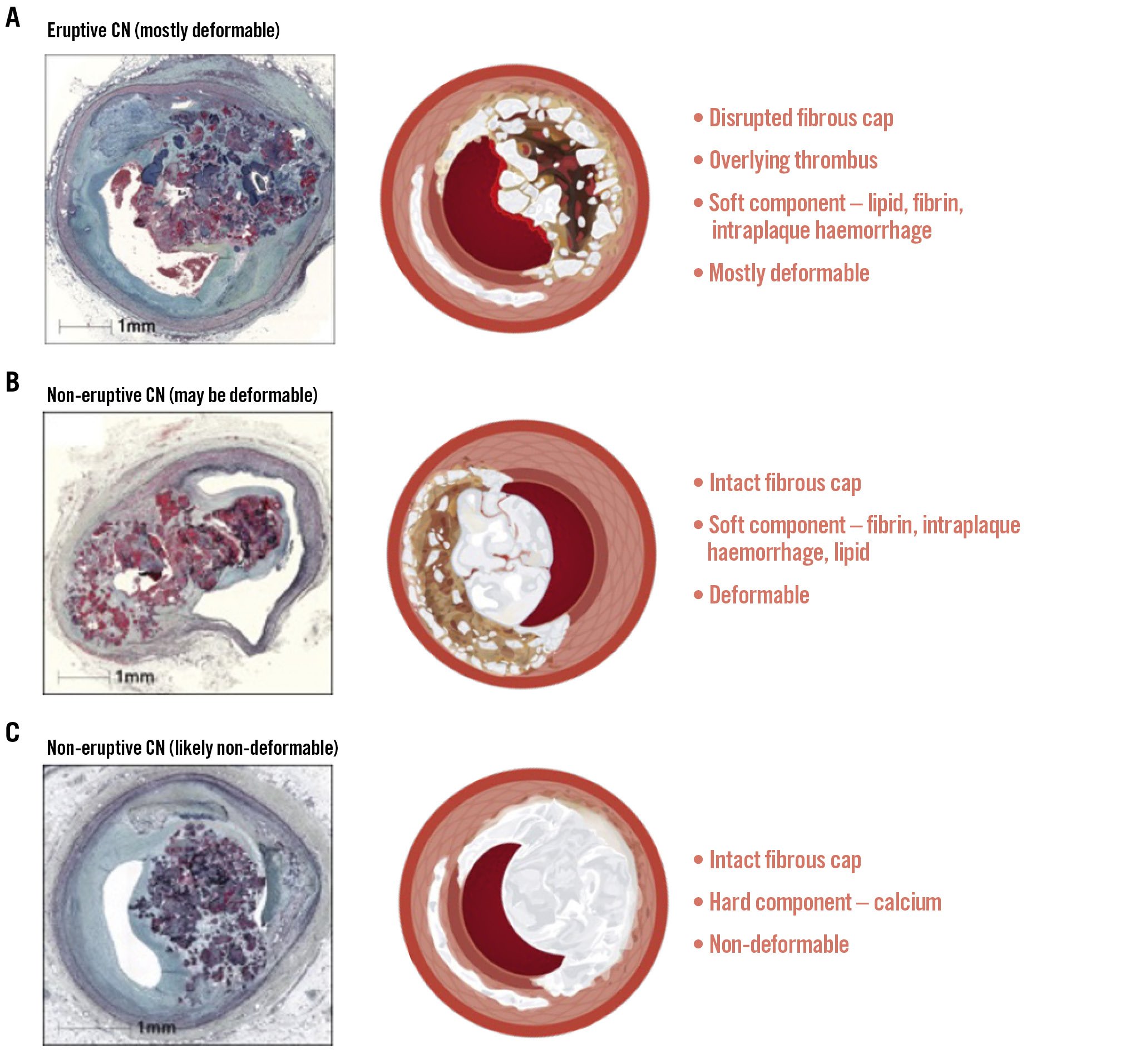
Central illustration. Subtypes and characteristics of CNs. Histology figures reproduced with permission from3. CNs are classified into two subtypes: eruptive (A) and non-eruptive (B, C). Non-eruptive CNs may be deformable (B) or non-deformable (C), likely based on their predominant components. CN: calcified nodule
Conflict of interest statement
Z.A. Ali reports institutional grants from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems Inc, Medtronic, OpSens Medical, Philips, and Shockwave Medical; personal fees from Amgen, AstraZeneca, and Boston Scientific; and equity from Elucid, Lifelink, SpectraWAVE, Shockwave Medical, and Vital Connect. A.V. Finn has received institutional research support from Abbott, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, Amgen, Asahi Intecc Medical, Avantec Vascular, BD, Biosensors, Biotronik, Bolt Medical, Boston Scientific, Concept Medical, Cordis, Dexcom, Edwards Lifesciences, Elucid Bioimaging, Endotronix, Envision, Filterlex, InterShunt Technologies, Invatin, Lahav, LimFlow, Medtronic, MicroVention, OrbusNeich, Protembis, Recor Medical, Regencor, Renata Medical, Restore Medical, Shockwave Medical, SMT, SpectraWAVE, Surmodics, Terumo, The Jacobs Institute, and Xeltis; and he has received honoraria from Abbott (stents), Boston Scientific (LAAO), Concept Medical, and Medtronic. J.C. Spratt has received research grants from Shockwave Medical; and has received consultant fees from Shockwave Medical and Boston Scientific. A. Maehara is a consultant for Boston Scientific, Canon, Amgen, and SpectraWAVE. D. Shin reports a research grant from SCAI/Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

