Abstract
Background: Calcified coronary lesions impede stent expansion and are associated with poor outcomes after percutaneous coronary interventions. Intravascular lithotripsy (IVL) has emerged as a safe and effective pretreatment to optimise stent implantation.
Aims: This study assesses the LithiX lithotripsy device, which uses the Hertz contact (HC) mechanism to fragment calcium while minimising injury to surrounding soft tissue, without requiring an external energy source.
Methods: The multicentre, prospective PINNACLE-I clinical trial enrolled patients with up to two moderately to severely calcified de novo lesions. The primary endpoints were <50% residual stenosis without in-hospital major adverse cardiovascular events (MACE) and the 30-day MACE rate. Clinical events were assessed up to 6 months.
Results: Sixty patients with 63 lesions were treated with successful delivery and use of the HC-IVL catheter and a median procedure time of 59.5 min (interquartile range: 40.5-76.0). The primary endpoint was achieved in 98.3%. All patients had residual stenosis <30% after stent placement. The 30-day MACE rate was 1.7%, due to 1 periprocedural target vessel non-Q-wave myocardial infarction. There were no cardiovascular deaths, no definite or probable stent thromboses, nor any device-related events up to 6 months of follow-up. The optical coherence tomography substudy in 32 subjects identified a wide range of calcium morphologies, including calcium arcs of 96-360° and calcified nodules. Following HC-IVL, calcium fractures were achieved in 90.6% of lesions, and the mean fracture depth was 0.81±0.33 mm. Stent expansion at the minimum stent area site was 96.7±25.5%.
Conclusions: PINNACLE-I demonstrated the feasibility, safety, and efficacy of the novel HC-IVL to fracture calcified lesions and achieve optimal stent expansion in a broad range of calcium morphologies.
Calcified coronary lesions remain a key challenge in percutaneous coronary interventions (PCI). Vessel calcification impedes stent expansion, increases the risk for malapposition, and is associated with increased complication rates and poor outcomes123. At present, nearly one-third of PCI involve lesions with moderate to severe calcification24.
Previous calcium treatment modalities, including off-label use of high-pressure balloons, cutting or scoring balloons, and atheroablative technologies (i.e., laser, rotational, and orbital atherectomy), have inherent limitations24. These include safety and effectiveness concerns (bulky device profiles hindering crossability, risk of vascular injury, and guidewire bias leading to eccentric ablation) and usability challenges (steep learning curves, high capital equipment costs, long procedure and fluoroscopy times, and minimum procedure volume requirements to maintain proficiency)24. In contrast, lithotripsy offers effective calcium fragmentation while minimising injury to adjacent non-calcified tissue through mechanical (manual) and energy-based modalities56. Energy-based intravascular lithotripsy (IVL) was developed after mechanical devices at the time failed to achieve effective calcium fragmentation without significantly injuring non-calcified adjacent vessel tissue or the vessel itself246. However, current energy-based IVL technologies require an external energy source and capital equipment for amplified stress generation78.
The LithiX Hertz Contact IVL (HC-IVL) System (Elixir Medical) is the first mechanical IVL platform without the need for an external energy source or capital equipment, offering an efficient procedure workflow and a potentially faster learning curve. It is based on the Hertz contact theory, which explains how discrete stress develops when a spherical surface deforms a planar object9. The parameters that influence the mechanism of amplified stress are contact force, the modulus of elasticity of the two contact surfaces, and the small, discrete area of contact. The novel application of Hertz contact theory to a balloon-based form factor led to the development of HC-IVL.
The PINNACLE-I clinical trial evaluated the feasibility, safety, and efficacy of HC-IVL for treating moderately to severely calcified coronary artery lesions.
Methods
Study design
PINNACLE-I is a multicentre, prospective, single-arm study conducted at 7 sites in Belgium and the Netherlands, which enrolled 60 patients. Thirty-two patients were scheduled for additional optical coherence tomography (OCT) assessment preprocedure, post-LithiX treatment, and post-stent implantation. Clinical follow-up was scheduled at 30 days and 6 months.
An independent core laboratory (Cardiovascular European Research Center) performed angiographic and OCT analyses, and an independent clinical events committee (RARAS Contract Research Organization) adjudicated all endpoint-related events. The study was conducted according to the Declaration of Helsinki, ISO14155, and local and national regulations, and was approved by the independent ethics committee of each participating centre and the respective regulatory authorities. All patients provided their written informed consent prior to any study procedure. The trial is registered at ClinicalTrials.gov: NCT05828173.
Participants
Patients requiring PCI in up to 2 de novo coronary artery lesions with moderate to severe calcification, reference vessel diameters between 2.25 mm and 3.5 mm, and a lesion length ≤34 mm were included. The full list of eligibility criteria is provided in Supplementary Table 1.
Interventions
The LithiX HC-IVL catheter is a mechanical lithotripsy device that consists of multiple low-profile metallic hemispheres, uniformly distributed and integrated across the surface of a semicompliant balloon. The hemispheres break the calcium directly or indirectly through application of physical forces targeting hard coronary calcification under low balloon pressure without removing the calcium. The Hertz contact mechanism provides exponential amplification of the discrete stress required for calcium fragmentation, eliminating the need for an external energy source (Central illustration, Figure 1, Moving image 1).
The delivery system is compatible with standard 0.014” (0.36 mm) guidewires, and the device crossing profile is compatible with a 5 Fr guiding catheter with an internal lumen diameter of at least 1.67 mm (0.066”). Size 6 Fr guiding catheters are recommended to facilitate the use of adjunct devices. The distal portion, including the balloon and hemispheres, is coated with a hydrophilic coating to enhance deliverability to the target location.
Available balloon nominal diameters range from 1.5 mm to 3.5 mm, with a nominal length of 14 mm. The nominal inflation pressure is 5 atm, and the rated burst pressure is 12 atm. The number of hemispheres per balloon ranges from 27 to 45 depending on the balloon diameter.
At the start of the study, predilatation was performed with a 1.5 mm LithiX device, thereafter upsizing to the reference vessel diameter. Over the course of the trial, the procedure was revised to a standard PCI workflow, and predilatation using a non-compliant balloon was required to assess resistance to lesion expansion due to calcification prior to the use of HC-IVL. The nominal size of the HC-IVL device was recommended to be approximately 0.25 mm smaller and not larger than a 1:1 ratio to the reference vessel diameter. The device was recommended to be expanded to at least nominal pressure, but no higher than the rated burst pressure. The workflow allowed multiple inflations within the same lesion to create new points of contact and cracks that propagate under sustained pressure during each inflation phase. Post-dilatation with a non-compliant balloon sized at a 1:1 ratio to the reference vessel diameter was recommended to optimise lesion preparation. The selection of stent size was left to the discretion of the investigators, and stent implantation was performed per standard practice.
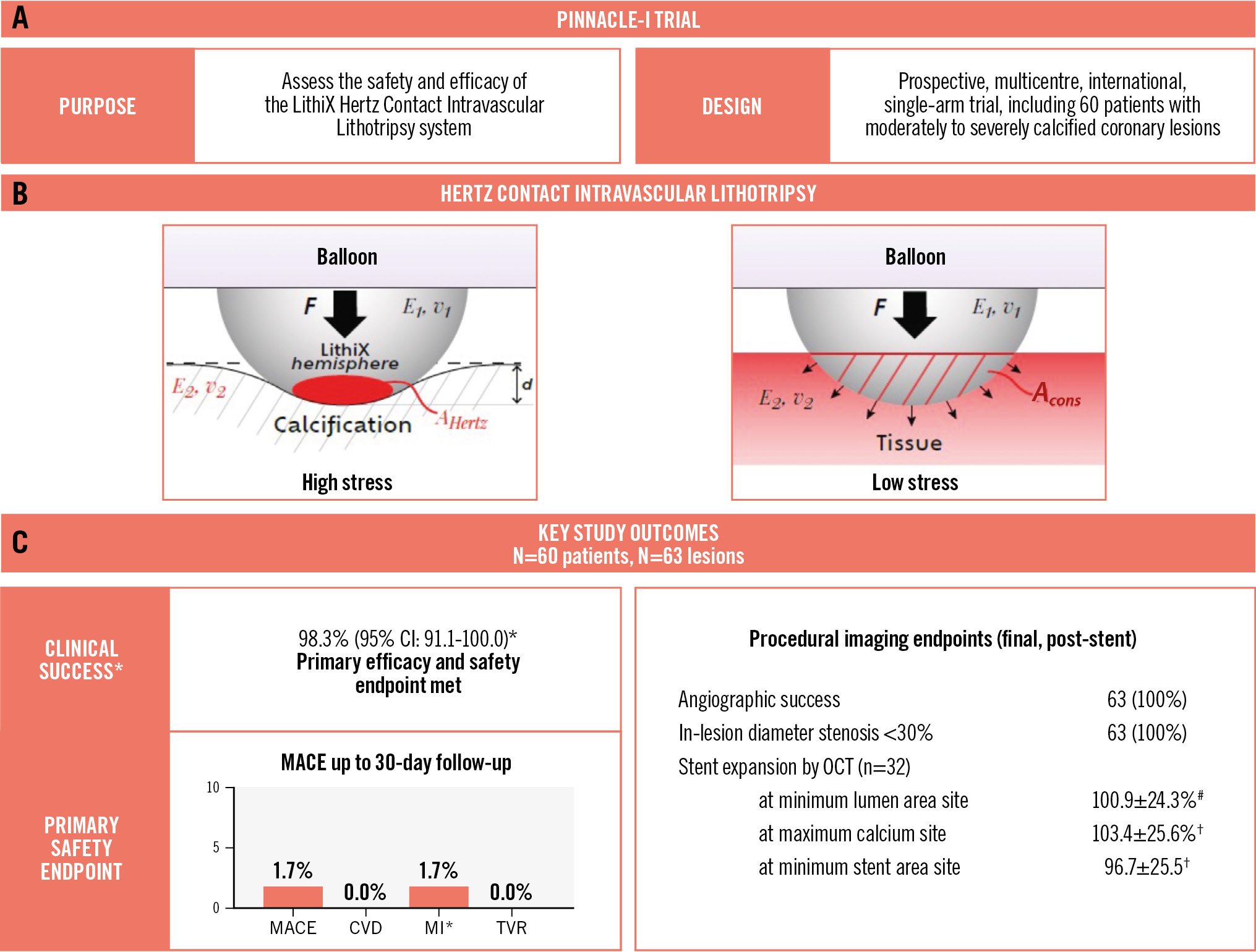
Central illustration. PINNACLE-I: treating calcified lesions with the Hertz contact mechanism. A) PINNACLE-I trial design. B) The Hertz contact theory describes the development of localised stress when a spherical surface deforms a planar object. Parameters that influence the mechanism of amplified stress are contact force (F), the effective modulus of elasticity of the two contact surfaces (E, v), and the area of contact at the interface (AHertz: discrete, small contact area with hard calcium; Acons: sustained, large contact area with soft tissue). C) Key study outcomes. MACE was a composite of CVD, MI, and TVR. *The primary efficacy and safety endpoint (clinical success) was achieved in all but one patient who experienced a periprocedural target vessel non-Q-wave MI. No angiographic complications were observed post-stenting, and all lesions had a final residual stenosis <30%. Optimal stent expansion was demonstrated by optical coherence tomography imaging (n=32). #n=29 lesions; †n=30 lesions. CI: confidence interval; CVD: cardiovascular death; HC-IVL: Hertz contact intravascular lithotripsy; MACE: major adverse cardiovascular events; MI: myocardial infarction; OCT: optical coherence tomography; TVR: target vessel revascularisation
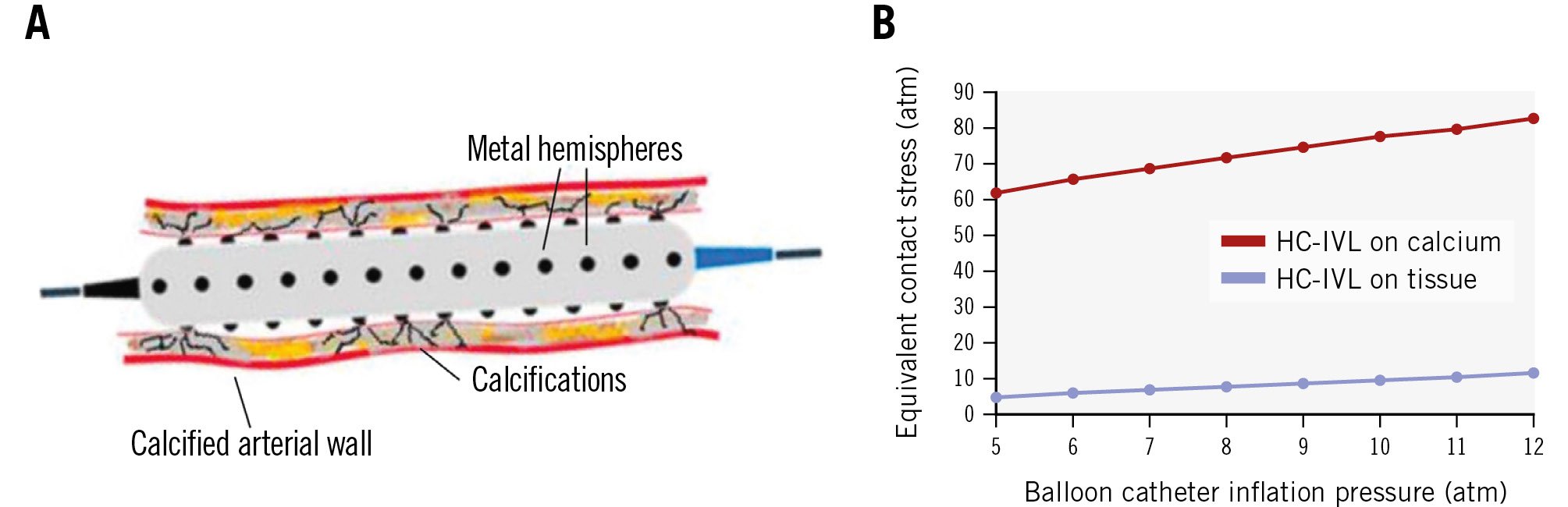
Figure 1. HC-IVL and its effects on tissue and calcium. A) The HC-IVL device consists of multiple low-profile metal hemispheres integrated along the surface of a semicompliant balloon. These hemispheres fragment calcium through exponential amplification of discrete stresses on calcified plaque while minimising stress on adjacent non-calcified tissue. B) Contact stress on calcium versus tissue. HC-IVL: Hertz contact intravascular lithotripsy
Endpoints and definitions
The primary effectiveness and safety endpoint was clinical success, defined as residual stenosis <50% after final treatment (with or without stenting) with no evidence of in-hospital major adverse cardiovascular events (MACE; a composite of cardiovascular death, myocardial infarction [MI], and target vessel revascularisation). An additional primary safety endpoint was the rate of MACE up to 30 days post-procedure.
Secondary endpoints included in-lesion angiographic success post-stenting, defined as success in facilitating stent delivery with <50% residual stenosis and without serious angiographic complications of the main branch (severe dissection impairing flow [type D-F], perforation, abrupt closure, persistent slow-flow, or no-reflow); stent delivery success; target lesion failure (TLF; composite of cardiovascular death, target vessel MI, and clinically indicated target lesion revascularisation [TLR]); MI (periprocedural MI defined according to the Academic Research Consortium 2 definitions10 and spontaneous MI according to the third universal definition11); target vessel revascularisation (any repeat PCI or surgical bypass of any segment of the target vessel); TLR; definite or probable stent thrombosis10; and mortality. Angiographic endpoints included acute gain and residual diameter stenosis (<50% and <30%), and OCT endpoints included the presence of calcium fractures, stent area, acute area gain, and stent expansion (stent area / average reference lumen area x 100).
Moderate to severe calcification is characterised by radiopacities visible before contrast dye injection during the cardiac cycle or without cardiac motion. The maximum continuous calcium arc corresponds to the arc of an individual calcium deposit from the cross-sectional image by OCT12. Eccentric calcium is defined as a calcium arc of ≤270°, whereas concentric calcification is defined as a calcium arc of >270°13. The procedure time is defined as the time elapsed from the insertion of the first guide catheter until the removal of the last guide catheter.
Statistical analysis
The primary safety and performance endpoint was compared against an objective performance goal of 80%. The objective performance goal was based on a review of the approved calcium modification devices reported in the literature1415.
The primary analysis is based on data available for the intention-to-treat population. The number of subjects, percentages, and Clopper-Pearson exact 95% confidence intervals (CI) were constructed for the primary endpoints. Continuous data are presented as mean and standard deviation or median and interquartile range (IQR) as appropriate, and categorical data as frequency counts and percentages. Missing data were not imputed. The statistical analysis of clinical data was performed using SAS, version 9.4 or later (SAS Institute).
Results
Baseline characteristics
From 27 April 2023 to 1 March 2024, 60 patients were enrolled. Patients were 72.1±6.8 years old, 81.7% presented with chronic coronary syndrome and 18.3% with acute coronary syndrome. Complex type B2/C lesions were present in 79.4%. Reference vessel diameter, minimum lumen diameter, and target lesion length were 2.79±0.47 mm, 1.09±0.34 mm, and 14.4±6.8 mm, respectively (Table 1).
The OCT substudy included 32 subjects. The average maximum continuous calcium arc by OCT was 263.4±77.1° with a minimum of 96° and a maximum of 360°. Calcium length was 23.9±6.6 mm. A total of 13 subjects had eccentric calcified lesions, 19 had concentric calcifications, and nearly one-third of all lesions presented with calcified nodules (n=9), per the core lab assessment (Table 2, Table 3).
Table 1. Baseline characteristics.
| Baseline characteristics | N=60 patients |
|---|---|
| Age, years | 72.1±6.8 |
| Sex | |
| Male | 39 (65.0) |
| Female | 21 (35.0) |
| Current smoker | 13 (21.7) |
| Hypertension | 46 (76.7) |
| Dyslipidaemia | 45 (75.0) |
| Diabetes mellitus | 18 (30.0) |
| Renal insufficiency | 9 (15.0) |
| Prior myocardial infarction | 12 (20.0) |
| Prior PCI | 17 (28.3) |
| Prior CABG | 4 (6.7) |
| Prior stroke | 5 (8.3) |
| Clinical presentation | |
| Acute coronary syndrome | 11 (18.3) |
| Chronic coronary syndrome | 49 (81.7) |
| Lesion characteristics | N=63 lesions |
| Target lesion classification | |
| A/B1 | 13 (20.6) |
| B2/C | 50 (79.4) |
| Target vessel | |
| LAD | 33 (52.4) |
| LCx | 5 (7.9) |
| RCA | 23 (36.5) |
| Protected left main | 1 (1.6) |
| Ramus | 1 (1.6) |
| Reference vessel diameter, mm | 2.79±0.47 |
| Minimum lumen diameter, mm | 1.09±0.34 |
| Diameter stenosis, % | 60.7±10.9 |
| Target lesion length, mm | 14.4±6.8 |
| Calcified length, mm | 25.1±11.9 |
| Calcification classificationa | |
| Severe | 38 (60.3) |
| Moderate | 25 (39.7) |
| Bifurcation with side branch involvement | 9 (14.3) |
| Data are displayed as mean±standard deviation or N (%). aSite assessed. CABG: coronary artery bypass graft; LAD: left anterior descending artery; LCx: left circumflex; PCI: percutaneous coronary intervention; RCA: right coronary artery | |
Table 2. Optical coherence tomography characteristics following Hertz contact intravascular lithotripsy (core laboratory).
| Preprocedure N=32 lesionsa | Following HC-IVL N=32 lesionsa | |
|---|---|---|
| Minimum lumen areab, mm² | 2.25±0.96 | - |
| Area stenosisb, % | 72.6±10.0 | - |
| Calcium length, mm | 23.9±6.6 | - |
| Maximum continuous calcium arc, ° | 263.4±77.1 | - |
| Range | 96°-360° | - |
| ≤270° | 13 (40.6) | - |
| >270° | 19 (59.4) | - |
| Presence of a calcified nodulec | 9 (31.0) | - |
| Calcium fracture | - | 29 (90.6) |
| 1 fracture | - | 5 (15.6) |
| 2 fractures | - | 12 (37.5) |
| ≥3 fractures | - | 12 (37.5) |
| Fracture depth, mm | - | 0.81±0.33 |
| Fracture width, mm | - | 0.66±0.29 |
| Data are displayed as mean±standard deviation or N (%). aOne subject had two target lesions but had OCT analysis on one target lesion only; bN=30 lesions; cN=29 lesions. HC-IVL: Hertz contact intravascular lithotripsy; OCT: optical coherence tomography | ||
Table 3. Optical coherence tomography characteristics at the sites of minimum lumen area, maximum calcium, and minimum stent area (core laboratory).
| Preprocedure | Final, post-stent | |
|---|---|---|
| At the site of minimum lumen area | N=30 lesions | N=29 lesions |
| Calcium angle, ° | 170.5±85.7 | - |
| Maximum calcium thickness, mm | 0.77±0.30 | - |
| Lumen area and stent area, mm² | 2.25±0.96 | 7.52±2.37 |
| Acute area gaina, mm² | - | 4.70±1.83 |
| Area stenosis, % | 72.6±10.0 | 11.2±14.2 |
| Stent expansion*, % | - | 100.9±24.3 |
| At the site of maximum calcium | N=30 lesions | N=30 lesions |
| Calcium angle, ° | 210.1±96.1 | - |
| Maximum calcium thickness, mm | 1.04±0.34 | - |
| Lumen area and stent areab, mm² | 4.86±2.46 | 8.34±2.29 |
| Acute area gainc, mm² | - | 3.87±2.47 |
| Area stenosis, % | 38.2±27.1 | 3.5±5.7 |
| Stent expansion*, % | - | 103.4±25.6 |
| At the site of minimum stent area | N=28 lesions | N=30 lesions |
| Calcium angle, ° | 125.8±91.9 | - |
| Maximum calcium thickness, mm | 0.72±0.43 | |
| Lumen area and stent area, mm² | 3.82±1.77 | 6.57±2.14 |
| Acute area gaina, mm² | - | 3.38±1.90 |
| Area stenosis, % | 51.7±22.3 | 14.2±12.7 |
| Stent expansion*, % | - | 96.7±25.5 |
| Data are displayed as mean±standard deviation or N (%). *Stent area / average reference lumen area x 100. Data were not available for all 32 lesions in each group. aN=28 lesions; bN=29 lesions; cN=27 lesions | ||
Procedural characteristics
Procedural details are provided in Table 4. The median procedure time was 59.5 min (IQR: 40.5-76.0), and the median fluoroscopy time was 13.1 min (IQR: 9.9-20.4). Predilatation prior to HC-IVL use was performed in 81% (51/63) of lesions, all lesions were treated with a stent, and post-stent dilatation was subsequently performed in 90.5% (57/63) of lesions.
Table 4. Procedural characteristics.
| N=60 patients | |
|---|---|
| N=63 lesions | |
| Access | |
| Radial | 52 (86.7) |
| Femoral | 8 (13.3) |
| Procedure time, min | 59.5 (40.5-76.0) |
| Contrast volume, mL | 180 (149-250) |
| Fluoroscopy time, min | 13.1 (9.9-20.4) |
| Number of LithiX devices used*a | 2 (1-2) |
| LithiX crossing success* | 62 (98.4) |
| Balloon rupture | 0 (0) |
| Device diametersa (N=104) | |
| 1.5 mm | 19 (18.3) |
| 2.0 mm | 6 (5.8) |
| 2.25 mm | 5 (4.8) |
| 2.5 mm | 19 (18.3) |
| 2.75 mm | 9 (8.7) |
| 3.0 mm | 26 (25.0) |
| 3.5 mm | 20 (19.2) |
| Predilatation* | 51 (81.0) |
| Number of stents implanted* | 1.2±0.5, 1 (1-1) |
| Post-dilatation* | 57 (90.5) |
| Data are displayed as mean±standard deviation, median (interquartile range), or N (%). Values are patient level unless otherwise indicated. *Lesion-level analysis. aTwelve 1.5 mm LithiX devices were used for predilatation. | |
Primary outcomes
The primary safety and effectiveness endpoint was met, with in-hospital clinical success achieved in 59 subjects out of 60 (98.3% [95% CI: 91.1-100.0]); all patients achieved angiographic success with residual diameter stenosis below 30%, and one patient had a periprocedural non-Q-wave MI caused by distal embolisation post-stenting.
This periprocedural MI was the only incidence of MACE up to 30 days of follow-up (1.7% [95% CI: 0.0-9.1]). Six months of follow-up was available for all patients, except for one patient who died of a non-cardiovascular cause (acute myeloid leukaemia). There were no cardiovascular deaths, no definite or probable stent thromboses, no unanticipated events reported, nor any additional TLF events up to 6 months (Table 5).
Table 5. Clinical events during the procedure and follow-up period.
| N=60 patients | |
|---|---|
| N=63 lesions | |
| Final, post-stent | |
| Serious angiographic complicationa | 0/63 (0) |
| Severe dissection (type D-F) | 0/63 (0) |
| Perforation | 0/63 (0) |
| Abrupt closure | 0/63 (0) |
| Slow-flow or no-reflow | 0/63 (0) |
| Thrombus | 0/63 (0) |
| Spasm | 0/63 (0) |
| Distal embolism | 0/63 (0) |
| 30 days | |
| Major adverse cardiovascular events | 1/59 (1.7) |
| Cardiovascular death | 0/59 (0) |
| Myocardial infarctionb | 1/59 (1.7) |
| Target vessel revascularisation | 0/59 (0 ) |
| Target lesion failureb | 1/59 (1.7) |
| Non-cardiovascular deathc | 1/60 (1.7) |
| Definite or probable stent thrombosis | 0/59 (0) |
| 6 months | |
| Target lesion failureb | 1/59 (1.7) |
| Cardiovascular death | 0/59 (0) |
| Myocardial infarctionb | 1/59 (1.7) |
| Clinically indicated target lesion revascularisation | 0/59 (0) |
| Target vessel revascularisationd | 1/59 (1.7) |
| Non-cardiovascular deathc | 1/60 (1.7) |
| Definite or probable stent thrombosis | 0/59 (0) |
| Data are displayed as n/N (%). aIn the main branch, one side branch occlusion without further complication was reported. bTarget vessel myocardial infarction (periprocedural, non-Q-wave). cAutopsy report concluded acute myeloid leukaemia with haemophagocytic lymphohistiocytosis. dOne subject had target vessel revascularisation (not target lesion), non-target vessel revascularisation, and non-target vessel myocardial infarction (spontaneous, non-Q-wave) | |
Imaging outcomes
The stent delivery success rate was 100%. Angiographic success post-procedure was 100% with no severe dissections, perforations, nor other angiographic complications involving the main branches. One subject had a side branch occlusion with no further complications. The angiographic acute lumen gain following HC-IVL was 0.79±0.40 mm and 1.60±0.48 mm post-stenting (Figure 2).
Calcium fractures post-HC-IVL were identified by OCT in 90.6% of lesions (2 or more calcium fractures in 75.0% of lesions) with a mean fracture depth of 0.81±0.33 mm. The final area stenosis was 11.2±14.2% at the minimum lumen area (MLA) site, 3.5±5.7% at the maximum calcium (MC) site, and 14.2±12.7% at the minimum stent area (MSA) site. Stent expansion at the MLA, MC, and MSA sites was 100.9±24.3%, 103.4±25.6%, and 96.7±25.5%, respectively (Table 2, Table 3).
In a post hoc analysis of the OCT subgroup, lesions were assessed by calcium morphology. The fracture depth was 0.76±0.28 mm in eccentric calcified lesions (N=13) and 0.85±0.36 mm in concentric calcified lesions (N=19). Stent expansion at the MLA site, the MC site, and the MSA site was 101.0±19.6%, 97.9±19.3%, and 101.4±23.7% for eccentric lesions and 100.8±26.9%, 106.6±28.7%, and 94.0±26.7% for concentric lesions, respectively (Supplementary Table 2, Supplementary Table 3). In lesions with calcified nodules (N=9), the average preprocedural calcium angle was 202.6±96.4°, and a stent expansion of 102.8±16.0% was achieved at the site of maximum calcium. Representative OCT images for eccentric and concentric calcified lesions and calcified nodules are provided in Figure 3.
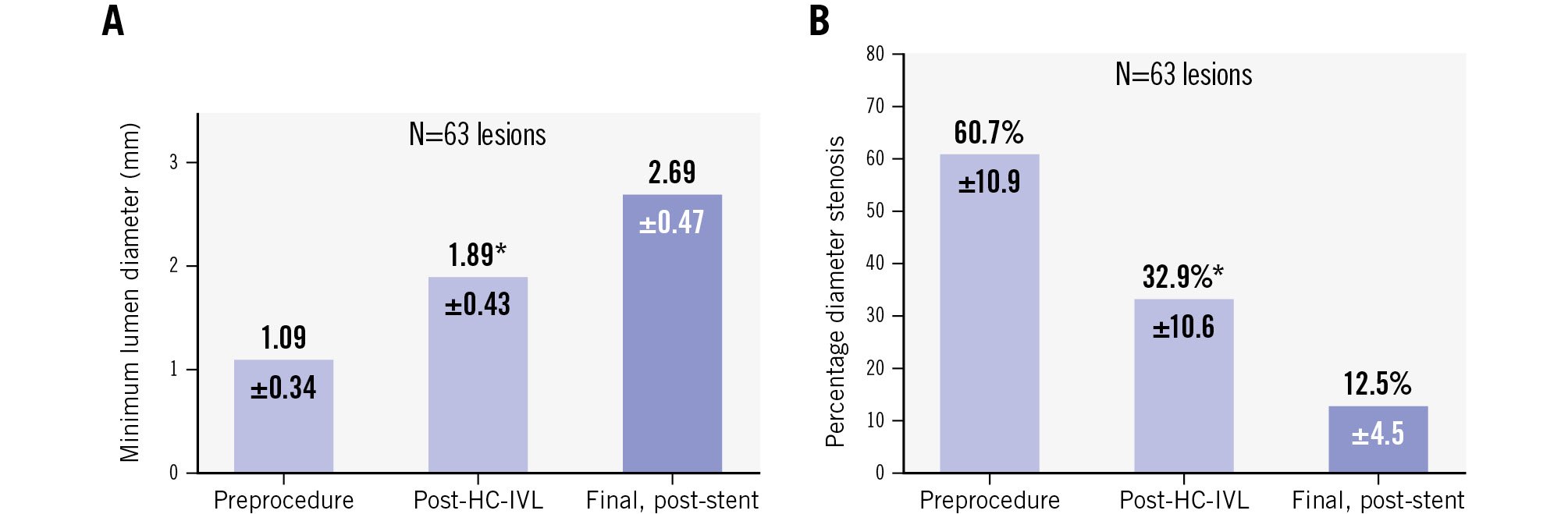
Figure 2. Angiographic outcomes after HC-IVL and stent implantation. A) Lumen gain: acute lumen gain post-HC-IVL was 0.79±0.40 mm and final, post-stent was 1.60±0.48 mm. B) %DS reduction post-HC-IVL and post-stent implantation confirms effective calcium fragmentation and vessel expansion. *N=55 lesions. %DS: percentage diameter stenosis; HC-IVL: Hertz contact intravascular lithotripsy
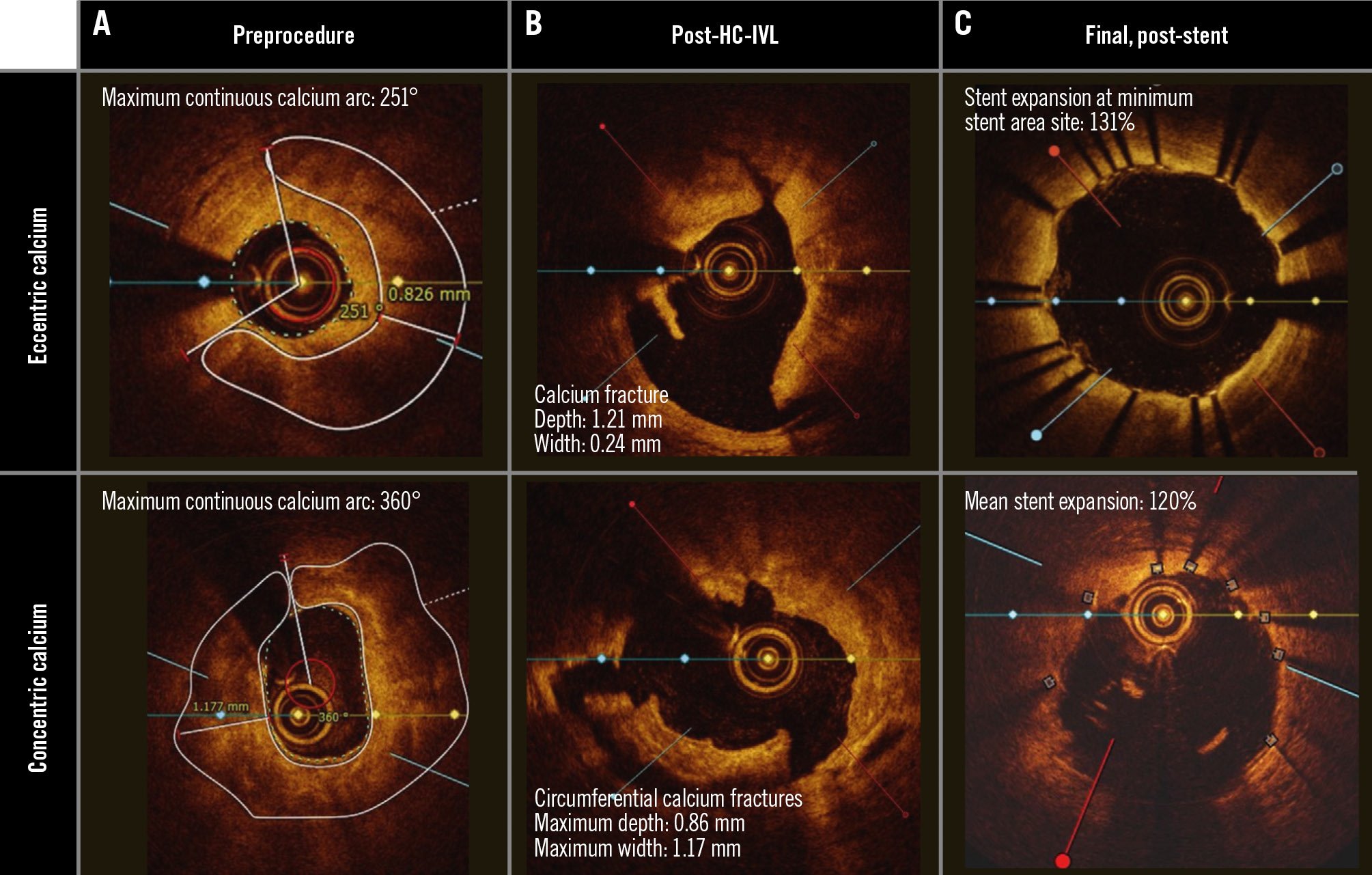
Figure 3. Optical coherence tomography of HC-IVL in eccentric and concentric calcified coronary plaques. A) Cross-sectional OCT images of representative eccentric calcified and concentric calcified coronary lesions prior to HC-IVL. The white line marks the calcium. B) Following HC-IVL, OCT images demonstrate calcium fractures. C) Following stent implantation, optimal stent expansion was achieved. HC-IVL: Hertz contact intravascular lithotripsy; OCT: optical coherence tomography
Discussion
PINNACLE-I assessed the safety and effectiveness of the HC-IVL device to fragment calcium for the treatment of moderately to severely calcified coronary lesions. The main observations from PINNACLE-I showed (i) effective calcium modification, with calcium fractures identified in more than 90% of lesions, with an average fracture depth of 0.81 mm across a wide range of calcium morphologies (eccentric lesions, concentric lesions, and calcified nodules); (ii) optimised % stent expansion and MSA regardless of calcium morphology or distribution; (iii) extremely low incidences of MACE and other adverse angiographic or clinical outcomes; and (iv) short procedure times, attributed to the efficient workflow and ease of use of HC-IVL.
The HC-IVL system was designed to achieve effective calcium fragmentation and to allow optimal stent expansion by applying discrete physical forces that selectively interact with calcium while being atraumatic to adjacent soft tissue, minimising vessel injury. The Hertz contact mechanism provides effective amplification of low balloon pressures and application of discrete stress directly or indirectly on hard calcified surfaces that are consistent with the fracturing stress developed by energy-based lithotripsy systems16 without requiring an external energy source. The multiplicity of uniformly positioned small hemispheres along the balloon surface allows operators to secure multiple contact points along the calcified lesion to achieve effective fragmentation and lesion preparation.
In the OCT imaging substudy, 90.6% of lesions demonstrated calcium fractures with an average fracture depth of 0.81±0.33 mm and a fracture width of 0.66±0.29 mm. The extension of calcium fractures into the medial layer provides evidence of HC-IVL’s effective mechanism of action. Calcium fractures resulted in optimal stent expansion of 103.4±25.6%, 100.9±24.3%, and 96.7±25.5% at the site of maximum calcium, minimum lumen area, and minimum stent area, respectively. Importantly, these results were consistent regardless of the extent of calcification (concentric or eccentric) or the presence of calcium nodules, which remain a challenge with existing calcium modification options17. Interestingly, no balloon ruptures were observed in PINNACLE-I, potentially owing to the uniform coverage by metal hemispheres which limit direct contact between the balloon and the calcium, thereby reducing the risk of balloon damage.
The degree of stent expansion in PINNACLE-I is particularly notable, given that achieving at least 80% expansion at the site of minimum stent area has been recommended by expert consensus3. The results appear at least comparable to those observed in the Disrupt CAD I-IV pooled analysis12.
The HC-IVL procedure appears to be safe, without major angiographic complications, with the exceptions of an isolated small side branch occlusion occurring without clinical sequelae, and one MACE event in the first 30 days (a periprocedural MI, 1.7%). Although the present cohort size is limited, this event rate compares favourably with other recent trials and a meta-analysis of multiple trials using calcium-modifying technologies during coronary intervention7161819. Furthermore, a potentially attractive aspect of HC-IVL is the lack of electromechanical transduction or myocardial electrical capture, which are common during electrohydraulic IVL procedures. The absence of myocardial capture mitigates the occurrence of associated hypotension observed with this phenomenon202122.
In addition, the rate of TLF at 6 months remained at 1.7%, with no additional TLF events observed between 30 days and 6 months. These salutary clinical outcomes demonstrate that the HC-IVL technology has a reassuring safety profile, attributed to its novel design that enables exponential force amplification through discrete contact points which selectively target the calcium while minimising injury to adjacent non-calcified vessel tissue, as well as a low-profile, trackable system.
Notably, despite all operators being first-time users, lesion crossing and clinical success rates were 98.4% and 98.3%, respectively, and procedure duration and fluoroscopy times were relatively short (mean total procedure time: 58 minutes; fluoroscopy time: 13 minutes), comparing favourably with previously published data on other calcium-modifying technologies7814161719222324. These findings suggest little or no “learning curve” associated with HC-IVL technology. Further, the high procedural efficiency of HC-IVL can be attributed to the small device profile, improved trackability, and the workflow not requiring capital equipment or an electrical source. Therefore, the efficient workflow and ease of use of the HC-IVL may improve productivity in the cardiac catheterisation laboratory, expanding patient access – especially in emergent and urgent settings – and enabling calcium fragmentation capabilities in smaller clinics and PCI centres, including those in rural areas.
Limitations
The main limitation of the present study is the lack of a randomised comparison to other devices. Furthermore, the outcomes are restricted to the patient population presented herein. Calcification severity was site assessed, and 39.7% of patients had moderate calcification. Importantly, intravascular imaging indicated substantial complexity even within these moderate cases, such as eccentricity and calcified nodules, and optimal stent expansion (>90%) was consistently achieved across MLA, MC, and MSA sites. Angiographic parameters after predilatation were not assessed. While the study was not designed to assess performance in specific risk groups, the results (100% angiographic lesion success) were encouraging and support device effectiveness.
Conclusions
In conclusion, PINNACLE-I demonstrates the safety and effectiveness of the novel HC-IVL device to facilitate and optimise coronary stent implantation through an efficient IVL workflow. Optimised stent parameters (% expansion, MSA) were obtained following HC-IVL, regardless of calcium severity, distribution, or the presence of calcified nodules. Further studies, including larger and more diverse patient populations with longer-term follow-up, appear warranted.
Impact on daily practice
The novel LithiX Hertz Contact Intravascular Lithotripsy catheter uses the Hertz contact mechanism to fragment coronary calcium without an external energy source. The outcomes presented herein demonstrate a reassuring safety and performance profile, with high procedural success, calcium fractures in more than 90% of lesions, and optimal stent expansion in a broad range of calcium morphologies. The platform’s workflow and ease of use may improve productivity in the catheterisation laboratory and enable broader patient access.
Acknowledgements
We thank Beatrix Doerr, medical writer, for her help in preparing the manuscript, which was funded by Elixir Medical.
Funding
The study was funded by Elixir Medical.
Conflict of interest statement
S. Verheye reports consulting fees and payment/honoraria from Elixir Medical and Shockwave Medical. V. Paradies reports grants from Abbott paid to her institution; honoraria from Elixir Medical, Abbott, Boston Scientific, Novo Nordisk, and SMT; support for attending meetings/travels from Novartis; is an EAPCI Congress Committee Chair; and is an ESC CPC member. P.A.L. Tonino reports research grants from Opsens and Biosensors; lecture fees from Medtronic; a patent issued for SAVI index; participates in the DSMB of the Urgent 2.0, Elite and Elite Venous stent studies; and is the Chair of the Dutch Working Group Transcatheter Heart Valve Interventions. D. Cottens reports consulting fees from Elixir Medical; and honoraria from Boston Scientific and Abbott. Z. Mehmedbegovic is an independent core laboratory specialist at CERC. P.C. Smits reports institutional research grants from Abbott, SMT, MicroPort, and Daiichi Sankyo; consulting fees from Abbott, AstraZeneca, Terumo, and MicroPort; payments/honoraria from Abiomed, Terumo, and MicroPort; and participates in the DSMB of the Protector, Legacy, and ASET Japan trials, in the global advisory board of Abbott, and the European advisory board of Terumo (the latter paid to the institution); and is a minor shareholder of CERC. M.-C. Morice is a shareholder of Electroducer; and a shareholder and CEO of CERC. J. Bennett reports consulting fees from Elixir Medical, Biotronik, and Boston Scientific; honoraria/speaker fees from Elixir Medical, Biotronik, and Medtronic; an institutional research grant from Shockwave Medical; and participates on the advisory board of Elixir Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. LithiX device – mechanism of action.

