Summary
Background: A 74 year old male with a history of postero-lateral myocardial infarction in 1993 and coronary bypass surgery in 1994 was referred to the outpatient clinic in a frail general condition with signs of dyspnoea at rest.
Investigations: Physical examination, electrocardiography, laboratory tests, coronary angiography, abdominal angiography, Echo-doppler, transesophageal echo.
Diagnosis: Aortic stenosis.
Management: Valve replacement.
How should I treat?
Presentation of the case
The patient concerned is a 74 year old male (172 cm, 68 kg) with a history of postero-lateral myocardial infarction in 1993, coronary bypass surgery in 1994 (LIMA on anterior descending artery, venous jumpgraft on intermediate branch, obtuse marginal and postero-lateral branch of the circumflex and a single vein graft on posterior descending artery of the right coronary). He had a known claudication for which he underwent a femoro-popliteal bypass followed by balloon angioplasty. In addition, he has a bilateral renal artery stenosis complicated by hypertension and non-insulin dependent diabetes.
When he was first seen at the outpatient clinic he was in a frail general condition with signs of dyspnoea at rest. His blood pressure was 120/70 mmHg, the heart rate was irregular with a frequency of approximately 90 beats/min. There were the typical signs of an aortic stenosis in addition to rales at the base of both lungs. The ECG disclosed atrial fibrillation with normal intraventricular conduction. Blood count and chemistry were normal except for a creatinine 155 µmol/l (glomerular filtration rate of 38 ml/min) and a haemoglobin level of 6.3 mmol/l.
Because of the general condition and signs of heart failure, the patient was directly admitted and treated with diuretics intravenously. Echo-doppler confirmed the presence of an aortic stenosis. The peak velocity over the aortic valve (CW Doppler transthoracic echo) was 4.0 m/sec (peak gradient 64 mmHg) in association with a moderately reduced left ventricular function and a grade 1-2 aortic, mitral and tricuspid regurgitation.
Following re-compensation, left and right catheterisation was performed to define further treatment. The coronaries were occluded but the arterial and veins grafts were patent. There was severe and calcified peripheral vascular atherosclerosis (Figure 1).

Figure 1. Severe and calcified peripheral vascular atherosclerosis.
The systolic pulmonary artery pressure was 52 mmHg, the cardiac output was 5.6 l/min. The logistic EuroSCORE was 47%.
On the basis of the clinical and anatomical variables described above, the patient was rejected for surgical valve replacement and transfemoral valve implantation whether with the CoreValve Revalving System™ (CRS) for which a 18 Fr sheath is needed – or, let alone, the balloon expandable Edwards Sapien valve for which a 22 or a 24 Fr sheath is needed – was considered impossible. Therefore, a transapical valve replacement with the Edwards Sapien valve was planned. Yet, this procedure was aborted on the basis of reassessment of the findings of transesophageal echo after the patient was intubated (Figure 2).
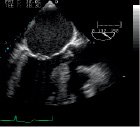
Figure 2. The transesophageal echo after the patient was intubated.
The decision to abort the procedure was taken by a multidisciplinary team that consisted of the treating cardiologist and cardiac surgeon, representatives of Edwards and the consulting cardiac surgeon acting as proctor. The decision to abort was based upon concerns of safety and feasibility of transapical valve implantation due to the septal hypertrophy in combination with the angled position of the LVOT in relation to the aortic root.
Given the poor outlook in terms of symptoms and survival, an angiogram of the left subclavian artery was performed in order to assess the feasibility of valve implantation via this artery. A stenosis in the subclavian artery in addition to a stenosis in the vertebral artery precluded this plan. (Figure 3)
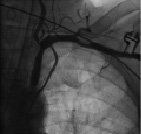
Figure 3. A stenosis in the subclavian artery in addition to a stenosis in the vertebral artery.
Also, valve implantation via the right subclavian artery proved to be no option because of severe calcification of this vessel.
The patient was then discussed with the vascular surgeon and interventional radiologist. The idea to surgically connect a temporary prosthetic conduit to the common iliac artery or distal aorta through which percutaneous valve implantation would be performed was discussed but not considered an option because of the extensive and almost circular calcification of the aorta-iliac tract rendering a safe and adequate suturing of such a conduit unlikely (Figure 4).
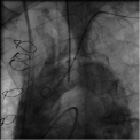
Figure 4. Circular calcification of the aorta-iliac tract.
How could I treat?
The Invited Experts’ opinion
This is a very complicated patient who offers about every challenge an interventionalist can imagine. His main problem is severe aortic stenosis in the presence of moderately reduced left ventricular performance, but there are also a number of comorbidities which make him a very poor, high risk candidate for surgery. In total, this is a desperate case and there will be no simple solution to it. Therefore, it is permissible to reconsider every strategy with the hope to find a loophole which allows correction of the dominant problem by taking a calculated risk.
Percutaneous CoreValve implantation: Figure 1 shows a diffusely calcified vascular tree in the pelvic area. Whenever possible, these patients should be avoided for percutaneous valve implantation because vascular complications are frequent, particularly with the Edwards SAPIEN valve which requires a 24 Fr sheath. However, most patients in our experience qualify for the smaller CoreValve system which requires only a 18 Fr sheath. Figure 1 gives us only a plane x-ray image, in this case I would like to see a CT angiography. The vessels seem to be of normal size, without any significant kinking, and the lumen may well exceed 6 mm, enough to permit passage of a 18 Fr sheath.
Subclavian artery route: Figure 3 shows an angiogram of the left subclavian artery; there is a 25-50% stenosis right before the origin of the vertebral artery, and a second stenosis more distal. The diameter of the subclavian artery at the level of the stenosis is slightly more than 6 mm according to our measurements based on markers on the pigtail catheter. There are no calcifications or irregular plaques in this vessel, therefore it can be expected that the vessel can be dilated slightly by the introduction sheath. I would exert great caution, but I see no strict contraindication in passing a CoreValve device through the subclavian artery.
Transapical approach Edwards SAPIEN Valve. Figure 2 gives us a TEE image of the LA and LV. There is significant septal hypertrophy just below the calcified aortic valve reaching 18 mm at its largest dimension. It is not advisable to use the transapical route for implantation of a valve, because it can be difficult to place a stiff guidewire into the aortic root and it is more difficult to negotiate the deployment sheath around the septal bulge. However, in patients where there are no alternatives, it is not impossible – and we have been successful in three patients with septal hypertrophy – to deploy a transapical Edwards SAPIEN valve with good results. Great care must be exercised not to entangle the guidewire with the chordae of the mitral valve. Once the prosthesis has negotiated the septal bulge and could be placed inside the aortic annulus, one should make certain that there is sufficient room between the septal bulge and the lower edge of the prosthesis. During the implantation process, one should expect some cephalad movement of the valve which can be counteracted by applying tension on the deployment sheath.
There is no guarantee that anyone of these various approaches will work, but by taking a calculated risk, the chances remain very good.
This is an interesting patient presenting with symptomatic aortic stenosis (AS) and high operative risk due to his comorbidities. Specific preoperative evaluation is mandatory in such patients, especially in presence of different therapeutic options: symptomatic AS per se is an indication for conventional surgical therapy in almost all patients with the exception of those that are frail, unmotivated or moribund. Rejection for surgical therapy, as well as the definition of a “non-surgical candidate”, remain a field of ongoing debate. We would consider most patients a surgical candidate, even if at high risk. The availability of transcatheter approaches for aortic valve implantation (AVI), using the transfemoral (TF) or transapical (TA) approach, however, is a valuable therapeutic option for high risk candidates. But despite all successes with these exciting new techniques1-5 cardiologists and surgeons have to keep in mind that there is not yet proof of superiority over conventional aortic valve surgery6.
In the patient presented here several factors become apparent: most importantly, a definitive judgement on the optimal approach can only be made after personally seeing an individual patient. This has to be kept in mind when discussing potential therapeutic options.
This patient definitively is a high risk candidate, he is 74 years old and has comorbidities such as moderately reduced ejection fraction, previous cardiac surgery with open grafts and severe peripheral vascular disease. We all know, however, that the EuroScore overestimates surgical risk, therefore this patients STS score risk, which may reflect the clinical situation better7, would be of interest.
Obviously TF-AVI was not considered a feasible therapeutic approach due to severe peripheral vascular disease and TA-AVI was scheduled in this patient. This definitely is an excellent approach to avoid any problems with the peripheral arteries. In addition, antegrade implantation of an aortic valve through the apex of the left ventricle using an anterolateral minithoracotomy is an intriguingly straight forward, simple and successful procedure. Little manipulation of the aortic arch is associated with very low stroke risk, which may be of benefit, especially in patients with peripheral vascular disease. Therefore electing TA-AVI would have been my primary choice. Preoperative screening would usually include transesophageal echocardiographic examination, especially for precise measurement of the diameter of the aortic annulus as well as to search for additional relevant pathologies. Septal hypertrophy, as present in this patient, is of some concern. More detailed information about the severity of septal hypertrophy would be warranted at this stage. Transcatheter AVI can be safely performed in patients presenting with moderate septal hypertrophy without having a significant gradient of the left ventricular outflow tract (LVOT). In the presence of a significant gradient in the LVOT, surgical myectomy in addition to conventional aortic valve replacement would be indicated. The TA approach allows for very precise valve positioning and thus may be advantageous in the presence of septal hypertrophy. During balloon valvuloplasty we should carefully watch for potential upwards motion of the balloon caused by the ventricular septum. In case of a stable balloon position during valvuloplasty, TA-AVI would have been the treatment of choice in this patient.
If transapical AVI cannot be performed at all and neither TF-AVI nor a subclavian arterial access are feasible, conventional aortic valve surgery should be considered, despite this patient’s increased operative risk. We should keep in mind that the patient is only 74 years old and suffers symptomatic aortic stenosis. He would benefit from a standard valve with proven longevity. Redo surgery in the presence of an acceptable ventricular function and cardiac output can be performed successfully by cardiac surgeons.
How did I treat?
Actual treatment and management of the case
It was decided to embark on a complex single stage procedure that consisted of percutaneous reconstruction of the right ilio-femoral tract with balloon angioplasty followed by the implantation of self-expanding stents.
In the case of vascular damage due to the dilatation, it was anticipated that it would be necessary to cover the entire ilio-femoral tract with an endograft. This procedure would be followed by the implantation of a CRS valve via an 18 Fr sheath inserted through the reconstructed ilio-femoral tract.
The implantation was performed under general anaesthesia and renal protection (prehydratation, 1,200 mg acetyl cysteine). As a standard procedure in our institution, arterial access using the Seldinger technique was done under ultrasonic guidance with a 7.5 mHz linear array probe to ensure vascular entry at a (relatively) calcium and plaque free part of the anterior wall of the common femoral artery and to avoid accidental puncture and sheath placement in the superficial femoral artery. A 10 Fr sheath was then introduced in the common femoral artery via a 0.038” Terumo wire followed by the insertion of a single Prostar XL 10 Fr system. This was done to place two suture wires ahead of the angioplasty of the femoral-iliac artery and valve implantation for purpose of vessel closure at the end of the procedure1. The Prostar system was removed over an 80 cm Amplatz extra stiff guidewire and the 10 Fr sheath was replaced. The suture wires were wrapped in swaps dripped in alcohol for reasons of sterility and secured with small clamps.
The femoral-iliac artery was then dilated with a balloon catheter followed by the implantation of a 94 mm long Wallstent with an unconstrained diameter of 10 mm. A number of balloon dilatations (balloon size 6, 7, 8 and 9 mm) in the stent were performed to ensure maximal expansion and to facility the introduction of the 18 Fr sheath needed for CRS implantation. Since this proved to be insufficient to advance the 18 Fr sheath, repeat dilatation with a 10 mm balloon was performed. Despite this, it still proved to be impossible to advance the sheath. A balloon expandable stent (Stent Express Vascular, length 57 mm, diameter 10 mm) was then placed in the proximal part of the common iliac artery. It nevertheless remained impossible to deliver the 18 Fr sheath. Kissing balloon dilatation with two Maverick XL 6.0x15 mm balloons was performed, first at the distal exit and then at the proximal entry side of the reconstructed vessel. Despite this, it was still impossible to advance the 18 Fr sheath. Given the risk of vessel rupture due to further dilatations, a 12x60 mm Fluency vascular mastent graft (BARD) was implanted which ultimately resulted in successful delivery of sheath and CRS system beyond the bifurcation of the iliac artery and to reach the aortic valve.
CRS implantation was then performed according to routine practice. A 29 mm inflow CRS was implanted. Because of aortic regurgitation grade 3, balloon valvuloplasty with a 25 mm balloon was performed. The residual aortic regurgitation at the end of the procedure was grade 1. There was no more gradient over the aortic valve. The femoral artery was closed by knotting the two Prostar wires that were already in place.
Because of a third degree AV block, a DDD pacemaker was inserted during further hospital stay. Echo-Doppler before discharge revealed a peak velocity of 1.2 m/sec (peak gradient 6 mmHg) with a grade 1 aortic regurgitation. The patient was seen at the out-patient clinic two months after the procedure. He was symptom free and had resumed his normal daily activities.
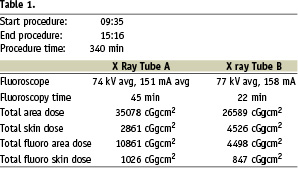
As written before, this technology and this procedure not only herald the inception of a new era in cardiovascular medicine. It also is the amalgamation of an enthusiastic collaboration between the industry and a dedicated, multidisciplinary group of physicians prepared to work together, to widen the boundaries, bringing innovation to clinical practice for the benefit of the patient irrespective of the constraints of either effort or cost1. The technical details of the procedure are summarised in Table 1.
Reference

