Product description
HBOC-201 (Haemoglobin glutamer-250 [bovine]; Hemopure®) is a polymerised, iso-oncotic, high-molecular weight, bovine haemoglobin-based oxygen carrier (HBOC) for intravenous infusion. HBOC-201 is manufactured by Biopure Corporation in Cambridge, Massachusetts, USA.
History
The development of HBOC-201 has been driven by concerns over blood safety and military interest in a product having improved storage and emergency use flexibility. HBOC-201 has been approved for the treatment of acute surgical anaemia in South Africa since 2001. Of late, needs beyond surgical anaemia, trauma and haemorrhagic shock have evolved to include ischaemic rescue, applicable to cardiology and vascular surgery.
Technical specifications
The manufacturing process involves: 1) purifying free haemoglobin (MW 65 kD) from bovine red cells by filtration and anion exchange chromatography, 2) polymerisation with glutaraldehyde, 3) fractionation to remove 65 kD haemoglobin, and 4) sterile packaging. The manufacturing process, approved by the FDA and European Medicine Evaluation Authority (EMEA), removes potential pathogens including bacteria, viruses, transmissible spongiform encephalopathy (TSE) and prions such as those believed to be responsible for Creutzfeldt-Jakob disease. Polymerisation with glutaraldehyde increases the molecular size (thereby increasing vascular retention) and stabilises the protein.
The finished solution is clear, deep purple and supplied in 250 ml units.
The technical characteristics of HBOC-201 are summarised in Table 1.

HBOC-201 has a circulatory half-life of 19 hours, (vs. approximately 0.5 hours for a similar amount of unmodified haemoglobin in animals). The P50 (partial pressure of oxygen at which the Hb is 50% saturated) is approximately 40 mm Hg1 (Figure 1) and permits a greater percentage oxygen off-load than that of red blood cells (RBCs), and other HBOCs characterised by lower P50 values.2
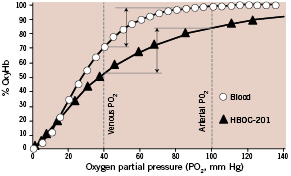
Figure 1. Haemoglobin dissociation curves for Hemopure and RBCs. At an arterial PO2 of 100 mm Hg, red cell haemoglobin is 98% saturated and Hemopure is 85% saturated. However, for a given decrease in PO2 (from 100 mmHg to 40 mmHg in this example) as blood travels from the lungs to peripheral tissues, the fraction of total oxygen released by HBOC-201 is equal to or greater than that released by RBCs to target organs. Vertical arrows represent the percent oxygen extraction between arterial and venous PO2 levels.
HBOC-201 has additional advantages over other oxygen therapeutics. Unlike HBOC products derived from human blood, HBOC-201 takes advantage of a plentiful supply of raw material (bovine haemoglobin). In addition, it has the highest haemoglobin concentration (13 g/dL) of any HBOC in clinical testing. This is a key advantage in maintaining oxygen delivery to ischaemic tissues with limited perfusion flow or in anaemia when early haemodilution with crystalloid or colloid solutions may complicate volume management. The smaller size of HBOC-201 polymers compared to a red cell and lower viscosity facilitate delivery of oxygen to remote tissues that may be poorly accessible by circulating erythrocytes. Unlike stored RBCs, which often carry the potential to elicit inflammatory responses and induce diminished host resistance3, HBOC-201 is free of pro-inflammatory stimuli and possible infectious agents. Finally, HBOC-201 is compatible with all blood types, is the only HBOC demonstrated to be stable at 40°C and has the longest room-temperature shelf life (three years) of all HBOCs currently in development.
Clinical experience
Summary of clinical experience
A total of 22 clinical trials evaluating the efficacy and safety of HBOC-201 have been completed with 826 subjects exposed to at least one dose of HBOC-201. Eighteen of the 22 studies were controlled, randomised trials. The clinical program was designed primarily to evaluate the extent to which HBOC-201 could replace red cell use or temporarily meet patient oxygen delivery needs in the setting of acute anaemia, an oxygen bridge™ for tissues until other definitive therapy was available. HEM-115, an elective surgery orthopaedic anaemia trial and the largest of the two phase III clinical trials, represents approximately 48% of all clinical trial subjects.
In four RBC-controlled studies, the median or mean number of allogeneic RBC units transfused was significantly lower for subjects randomised to HBOC-201 than in subjects randomised to RBCs. The mean ±SEM number of RBC units administered to HBOC-201 and RBC treatment groups, respectively, in HEM-115 were 1.4±0.1 and 3.1±0.1 (p<0.001).4 For HBOC-201-treated patients who eventually received RBCs, there was a significant delay in the time to the first RBC infusion with 75% of the HBOC-201 patients requiring their first allogeneic RBC transfusion four days after their first HBOC-201 infusion.
HBOC-201 has also been evaluated in subjects with cardiovascular disease. In the phase II COR-0001 trial, HBOC-201 was infused intravenously into subjects with stable angina and non-ST-segment elevation acute coronary syndrome (ACS) scheduled for elective PCI.5 Consistent with this drug’s well-known vasoconstrictive properties, HBOC-201 elicited a transient, mild increase in mean blood pressure accompanied by a slight decrease in cardiac output, but had no effect on left ventricular stroke work index (LVSWI) or systemic oxygen consumption. Increases in blood pressure were readily addressed, when appropriate, by standard antihypertensive drugs.
Using a novel preparation and administration route, oxygenated HBOC-201 was infused into the coronary arteries of subjects characterised by silent ischaemia, stable angina or unstable angina (Braunwald class I-IIIB)6 in a pilot phase II study (COR-0002) designed to determine if direct perfusion of the distal vessel with oxygenated HBOC-201 could mollify or avert ischaemia induced by total LAD coronary artery occlusion.7 Patients first received either intracoronary HBOC-201 during a brief coronary occlusion (test treatment) or coronary occlusion in the absence of intracoronary infusion (control treatment). After completion of the first intervention and a 20-min recovery period, patients crossed over to the second intervention. Control coronary artery occlusions induced deterioration in both systolic and diastolic LV function and haemodynamic parameters and caused ST segment elevations (all P< 0.05 vs. baseline). In contrast, HBOC-201 infusion prevented these changes, and while arrhythmia and angina required premature termination of all control occlusions, this did not occur with HBOC-201 infusions.
Expected physiological changes and side-effects
Clinical trials have identified adverse events (AEs) and side effects following treatment with HBOC-201. These effects include oliguria, increased blood pressure, nausea/vomiting, diarrhoea, abdominal pain and distension, dysphagia, flatulence, skin discoloration, scleral discolouration, decreases in pulse oximetry O2 saturation and haematocrit measurements, transient increases in methaemoglobin and increases in hepatic and pancreatic enzymes. HBOC-201 is eliminated via the reticuloendothelial system which metabolises the breakdown products of protein and heme. The heme is metabolised into bilirubin or recycled into red cell production.
Product safety
A full toxicology profile was completed for HBOC-201, including general toxicity (acute and repeat dose), cardiotoxicity, genotoxicity, reproductive toxicity, immunotoxicity, and renal toxicity studies. Overall, the data suggest that HBOC-201 has an acceptable toxicity profile.
Evaluation of HBOC-201 safety was performed on the results from 21 trials, including 797 subjects exposed to at least one dose of HBOC-201. The number of deaths (39, 2.7%) in the HBOC-201 group was not significantly different from mortality in the control groups (25, 2.1%).
Most incidents of increased blood pressure appear with greater frequency in the HBOC-201 groups than the comparators, are transient, mild and do not require treatment. Increases in blood pressure of sufficient amplitude to warrant treatment were captured as severe AEs (SAEs) and occurred with an incidence indistinguishable from that in control subjects.
Analysis of the potential for HBOC-201 to induce immunogenicity has been analysed in 473 HBOC-201 subjects and 418 controls across 14 clinical trials. The risk of immunogenicity was found to be exceedingly small (0.2% of subjects dosed with HBOC-201).
The serious adverse events occurring in the HEM-115 study have been analysed, including a search for their root causes.4 In HEM-115, the SAE incidence (0.34 SAEs/patient) was slightly higher in patients randomised to HBOC-201 than in patients randomised to packed red blood cells (PRBCs) (0.25 SAEs/patient) (P=0.062). The severity of anaemia correlated with an increased occurrence of SAEs, independent of treatment arm (P<0.001). Moderate or high need patients were matched between treatment arms to effect a valid safety comparison. Of those patients randomised to HBOC-201, a subset (designated “HR”) of the most severely anaemic were, per protocol, “crossed over” to receive PRBCs (Table 2).
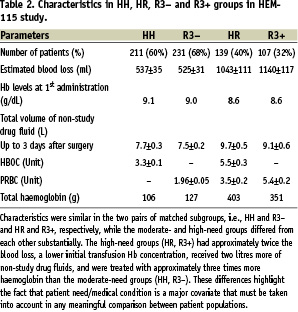
All other patients randomised to HBOC-201 (designated HH) were adequately treated with study drug alone. The HH subgroup, representing moderate need subjects, was matched to a subgroup of PRBC subjects who were adequately treated with ≤3 units PRBCs (R3–). The high-needs HR subgroup was compared to a corresponding high-needs PRBC subgroup (R3+), those that received >3 units.
The death rate and SAEs per patient were identical in groups HH and R3- (Table 3).
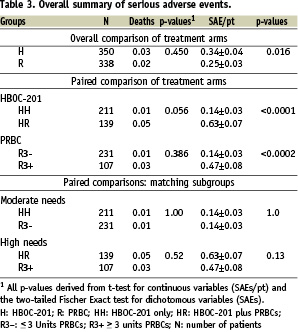
Likewise, the death rate and SAEs per patient in groups HR and R3+ were similar. However, comparisons of the two levels of clinical need within each treatment arm indicates that SAEs/patient are 4.5 fold higher in HR than in HH (P<0.0001) (Table 3). Also, SAEs/patient are 3.4 fold higher in R3+ compared to R3- (P<0.0002). Patients randomised to treatment with HBOC-201 had lower total haemoglobin (THb) concentrations, with subjects in the HR group having the lowest THb of the need-based subgroups. Under-treatment (i.e., persistent anaemia, P=0.035), age (P=0.046) and pre-existing cardiac disease (P=0.004) were covariant predictors of cardiac SAEs. By comparison, randomisation to HBOC (P=0.603) had no value as a predictor of cardiac SAEs. Fluid mismanagement (volume overload) also contributed to the incidence of SAEs. These results indicate that patient care, and in particular, failure to adequately treat anaemia, rather than toxicity, explains the disproportionately high incidence of SAEs in patients randomised to HBOC-201.8,9
Indications for use
Elimination of the need to cross-match and a three-year shelf life at room temperature make HBOC-201 well-suited to a variety of field indications including civilian and military pre-hospital trauma with haemorrhagic shock. Extensive preclinical evaluation of HBOC-201 as a resuscitative fluid has demonstrated superior efficacy over crystalloid and colloid fluids in treating this indication.10-12
Encouraging results from several preclinical studies11,13-15 warrant further exploration of the potential therapeutic effects of HBOC-201 in specific ischaemia-related pathologies including peripheral limb ischaemia, myocardial infarction (Figure 2) and stroke.
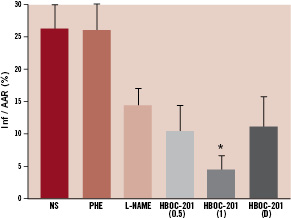
Figure 2. Total infarct size after HBOC-201 infusion in a canine myocardial infarct model. Ischaemia was induced by occluding the LAD coronary artery to achieve an 85-90% flow reduction. After 15 min of ischaemia, each animal was randomised to the following intravenous therapies: HBOC-201 (0.5 g/kg, n=6), HBOC-201 (1 g/kg, n=6), normal saline (NS) (7 ml/kg, n=6), phenylephrine (PHE) to achieve an increase in MAP similar to that induced by HBOC-201 (n=6), L-NAME (0.1 ml/kg/min for 2 min, then at 0.3 ml/kg/min thereafter, n=6) or HBOC-201 (1 g/kg, n=5), delayed (D) until 60 min after the onset of ischaemia. The LV was paced at a rate 10% higher than the spontaneous heart rate beginning 15 min after induction of ischaemia. Ischaemia was maintained for a total of 195 min, at which point pacing was stopped, followed by reperfusion for 180 min. Staining for myocardial area at risk (AAR) and infarct area (Inf) was performed at the conclusion of reperfusion. Total infarct sizes expressed as Inf/AAR were significantly reduced after HBOC-201 (1 g/kg) compared with NS and PHE (*P < 0.05). From reference 15, modified with permission.
Although known to induce small to modest increases in blood pressure, HBOC-201 vasoconstriction activity appears to be limited primarily to skeletal muscle vascular beds and the pulmonary circulation and does not involve the coronary, cerebral or splanchnic vasculature.16 Consistent with these preclinical data, HBOC-201 had no effect on coronary blood flow or coronary artery tone in COR-0001 trial subjects.5 The COR-0001 and COR-0002 clinical trials suggest that intravenous and intracoronary HBOC-201 is well-tolerated in patients with coronary artery disease. These results support the rationale for evaluating the efficacy of HBOC-201 against infarct development in ACS patients with ST segment elevation myocardial infarction (STEMI). HBOC-201 could be administered intravenously to a patient sustaining a myocardial infarction and/or via intracoronary infusion in the oxygenated state as an adjunct therapy to PCI, particularly when lesion access is difficult or when microvascular pathology contributes to low thrombolysis-in-myocardial-infarction (TIMI) coronary blood flow following successful treatment of a proximal coronary obstruction. The small particle size and low viscosity of HBOC-201 may bestow advantages to treatment of the low- or no-reflow patient over reperfusion with blood alone. An estimated 80,000 cases of post-revascularisation low (< 3) TIMI flow are realised annually in Europe.17
The efficacy of oxygenated HBOC-201 in preventing downstream ischaemia during coronary intervention and favourable optical transmission properties18 are consistent with using HBOC-201 as an adjunct therapy with intravascular imaging modalities (Raman spectroscopy, laser speckle imaging, infrared microscopy) that require temporary displacement of blood from the target field. Intermittent or continuous arterial perfusion with oxygenated HBOC-201 may also effectively maintain the viability of organs undergoing surgical repair distal to an arterial cross-clamp or in maintaining donor organ viability during transport to a recipient. Relevant to these indications, enrolment is nearly complete in a 60-subject clinical trial designed to determine the safety and efficacy of HBOC-201 when infused prior to initiating cardiopulmonary bypass for patients undergoing coronary artery bypass surgery.
Cardiogenic shock is an indication for which HBOC-201 may be especially well suited. Cardiogenic shock develops in response to acute myocardial infarction, leading to low cardiac index, low blood pressure and vital organ underperfusion. Cardiogenic shock is further characterised by a proinflammatory response involving upregulation of iNOS and inappropriately high levels of nitric oxide (NO). Excessive NO expression exacerbates both weak cardiac function and low blood pressure. As a putative NO scavenger, HBOC-201 would oppose the NO contribution to cardiogenic shock and restore blood pressure while facilitating oxygen delivery to ischaemic myocardium and other organs.19
Recommendations for management of patients receiving HBOC-201 (tips for use)
Although rare, increases in systolic blood pressure above 180 mmHg should be treated with standard antihypertensive drugs; successful treatment with nitroglycerin, nitroprusside and calcium channel blockers has been demonstrated. Because of plasma volume expansion, haematocrit is expected to decrease. Given the colloid properties of HBOC-201, attention to fluid management is necessary to avoid volume-related adverse events.
The data do not support the need for special monitoring of metHb in most patients treated with HBOC-201 unless also receiving one or more agents (e.g. benzocaine, lodocaine, prilocaine, dapsone, amyl nitrite, isobutyl nitrite, nitroglycerin, and primaquine) known to induce formation of methaemoglobinaemia. However, patients who receive higher doses of HBOC-201 and/or are intravascularly depleted prior to HBOC-201 infusion (e.g., trauma patients with massive bleeding) may have larger increases in metHb or may tolerate low metHb levels less well, and should have metHb levels monitored closely.
Summary
Clinical and preclinical studies have revealed a diverse array of indications in which the effectiveness of HBOC-201 has been demonstrated or appears likely. Included among these are indications involving cardiac and peripheral ischaemia in which this oxygen therapeutic may prove to be an important tool in the armamentarium of the cardiologist and surgeon. Preclinical studies and clinical trials are under way to further delineate and optimise the role of HBOC-201 as an oxygen therapeutic in cardiovascular medicine.
Acknowledgements
Source of funding: Certain preclinical studies and clinical trials reviewed in this document were supported in part or in full by grants from Biopure Corporation.

