Abstract
Aims: Haemoglobin based oxygen carriers (HBOCs) are considered in the treatment of patients with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI). In light of their potential vasopressor and colloidal properties, their effect on coronary physiology, safety and tolerability needs to be established.
Methods and results: In this phase II pilot trial, 45 patients were randomly assigned, (1:1:1) to double blind treatment with a 30 minute intravenous (IV) infusion of either 15 or 30 g of HBOC-201, compared to an equivalent volume of non-oxygen carrier colloid control. Systemic, pulmonary, and coronary haemodynamics were studied during this infusion period. IV HBOC-201 administration produced an increase in systolic blood pressure (SBP), pulmonary capillary wedge pressure and calculated systemic vascular resistance (SVR) and a concomitant decrease in cardiac output (CO); there was a decrease in mixed venous saturation (SVO2) following IV HBOC-201. The left ventricular stroke work index (LVSWI) was not altered by HBOC-201 treatment. Of note, no coronary vasoconstriction was observed, nor were there significant changes in resting average peak velocity (APV), coronary-artery diameter, volumetric coronary blood flow, or coronary vascular resistance. The percentage of patients with adverse events did not differ between the HBOC-201 treated and control groups (76% vs. 63%, respectively, P=0.49). Seven serious adverse events (SAE) occurred in six patients in the treatment group and two in two patients in the control group. Only one SAE (hypertension) was judged HBOC-201 related. Patients in both the HBOC-201 and control group had a similar incidence of increased liver alanine transaminase (31% vs 31%, respectively, NS); 10% of the patients in the HBOC-201 group had increases greater than three times the upper limit of normal. Differential increases were noticed in some inflammatory markers (IL-6, CRP) 18-24 hours after infusion between the HBOC-201 arms and the control group.
Conclusion: No compromise in the coronary blood flow or LVSWI was observed despite HBOC-201’s known vasoactive effects. One SAE was adjudicated as “drug related” and fully resolved. The clinical relevance of the differential rise in certain biochemical markers and the adverse effects of plasma haemoglobin in the context of ACS needs further investigation.
Introduction
Prompt reperfusion of ischaemic myocardium is the major focus of acute treatment of patients with ST-segment elevation myocardial infarction (STEMI). With (primary) PCI emerging as the new gold standard of ACS reperfusion therapy, new questions are arising about the best pharmaco-invasive strategy to limit the amount of myocardial damage occurring during the ischaemia and early reperfusion periods. Because of their ability to deliver oxygen, HBOCs have been considered for use in the treatment of ACS.
HBOC-201 is a cell-free polymerised bovine-haemoglobin solution in a balanced salt solution. HBOC-201 may act as a direct tissue oxygen donor and an “oxygen bridge” between RBCs and tissues1,2, facilitating oxygen transport from erythrocytes through plasma to the endothelium and organs and eventually to post-stenotic areas where plasma oxygen transport can improve tissue oxygenation.3 HBOC-201 can be stored at room temperature for a period of up to three years. In a dog myocardial ischaemia-reperfusion model, infusion of HBOC-201 prior to coronary artery occlusion reduced myocardial infarct size.4,5
The current study is the first attempt at introducing HBOC-201 in the treatment of ACS and addresses safety issues in the controlled setting of elective PCI.
Methods
Study design
The COR-0001 trial was a randomised 3-arm (1:1:1), double-blind, placebo-controlled, dose-finding pilot (phase II) study designed to investigate the safety and tolerability of IV HBOC-201 versus an equivalent amount of artificial colloid in patients with stable angina and non-ST-segment elevation (NSTE) ACS scheduled for elective PCI.
The study had one control arm and two active treatment arms with HBOC-201 delivered at different doses. In one arm 230 ml HBOC-201, equivalent to 30 g bovine Hemoglobin (Hb) was infused over 30 minutes. In the second arm 115 ml of HBOC-201 was infused over 15 minutes, at the same infusion speed of the first arm; thus delivering 15 g of Hb, sequentially followed by a 115 ml infusion of Voluven-Fresenius (a colloidal volume expander chosen for its molecular weight, similar to that of the study drug). The control arm consisted of 230 ml Voluven-Fresenius infused over 30 minutes. Randomisation was stratified by clinical site, using permuted blocks of six patients.6 Patients were allocated to a treatment by a central allocation telephone service.
Patients, investigators and the members of the Data Safety Monitoring Board (DSMB) were blinded to the treatment allocation during the study period. For blinding purposes in the catheterisation laboratory a double dummy technique was used (see online-only Data Supplement for details). The study was performed under Medical Ethics Committee approval and in accordance with the Declaration of Helsinki.
Patient population
The patients were enrolled in five centres in The Netherlands, Germany and Belgium selected for their expertise in cardiac physiology studies (Appendix 1, online-only Data Supplement).
Patients were eligible for the study if they had either unstable angina or NSTE ACS and had a severe stenosis in at least one coronary artery eligible for PCI. All patients had to provide written informed consent. Major exclusion criteria were: significant haemodynamic compromise requiring inotropic or vasopressor support, significantly altered left ventricular function (ejection fraction <35%), severe hypertension (>180/110 mmHg) not adequately controlled by antihypertensive therapy at time of study entry, renal impairment (serum creatinine >1.6 mg/dl) or contra-indications to the use of adenosine and/or standard drugs for coronary intervention and coronary artery disease. The patient weight at inclusion was limited to a maximum of 110 kg.
Study procedures
The catheterisation laboratory procedure was divided into three consecutive phases: the baseline, the study drug infusion period (230 ml solution/30 minutes) and the index PCI procedure. Haemodynamic monitoring and control angiography were performed at baseline and at three consecutive time points (10’, 20’, 30’) during the study drug infusion period. PCI, including adjunctive therapies, were performed according to standard institutional practices. No standard medications used in the management of patients with ischaemic heart disease were withheld by the study protocol, stopping rules on study drug infusion were predefined (online-only Data Supplement).
Safety endpoints and assessments
The primary endpoint of the study was the in-hospital safety assessment including systemic and coronary haemodynamics, thrombotic events, untoward drug interaction effects, allergic reactions, drug-dye interactions, met-haemoglobin formation, serious adverse events, as well as biochemical markers of inflammation, myocardial necrosis, renal and hepatic function. The extent of deviation of blood values beyond the limits of normal was graded by the DSMB/CEC members. Calculated measurements of eGFR (estimated glomerular filtration rate) by the Cockroft-Gault equation were used for estimating and reporting renal dysfunction.7
Additional analysis included a 30-day clinical follow-up, death (all-cause mortality), recurrent myocardial infarction, recurrent myocardial ischaemia and serious adverse events.
Patient symptoms and adverse events were evaluated by the study investigators using a graded severity index.8 An independent Data Safety Monitoring Board/ Clinical Event Committee (DSMB/CEC) reviewed aggregate safety data (including blood values) to identify potential patient safety issues. Safety monitoring and adjudication of clinical events with respect to their clinical relevance was performed by this committee. The data as classified by the DSMB/CEC was used in the final safety analysis unless otherwise specified.
A doppler steerable guidewire (0.36 mm [0.014 in.] in diameter) (Flowire, Volcano Corporation, Rancho Cordova, CA, USA) was positioned in a reference coronary artery without any significant stenosis and was coupled to a real-time spectrum analyser and video cassette recorder. Coronary flow velocity and coronary flow reserve measurements were performed at baseline and after the study drug infusion period in the first 30 consecutive study patients. To assess coronary flow reserve (the ratio of peak hyperaemic velocity to average peak velocity at base line), maximal hyperaemia was induced with peripheral IV infusion of adenosine (140 µg/kg/min).9 Each measurement was duplicated to check for consistency. Coronary blood flow was calculated as follows: (the average peak velocity ÷ 2) x the cross-sectional area of the coronary artery, calculated as πx (diameter of the artery ÷ 2)2, which assumed a time-averaged parabolic velocity profile and a cylindrical coronary artery.10 Coronary vascular resistance (in mmHg/ml/min) was calculated for the reference vessels as the mean arterial pressure divided by the coronary blood flow. The coronary vascular resistance index was calculated as the average peak velocity (APV) hyperaemic divided by the mean arterial pressure at rest.
Quantitative coronary angiographic assessments of the vessel segment comprising the flow wire as well as the coronary diameter at the tip of the Doppler wire, (between two side branches), were performed by an independent core laboratory (Cardialysis, Rotterdam, The Netherlands) with the use of edge-detection techniques.11
Systemic haemodynamic measurements included arterial blood pressure, recorded from a 7 Fr guiding catheter in the ascending aorta; pulmonary-artery and capillary wedge pressure (measured from the distal port of a 7 Fr Swan-Ganz catheter) and right atrial pressure (measured from the proximal port of the Swan-Ganz catheter). The heart rate and cardiac output, determined by thermodilution, were also recorded. Standard haemodynamic formulas were used to calculate systemic and pulmonary vascular resistance and their indexes.
Statistical analysis
Continuous baseline characteristics were analysed with one way analysis of variance and categorical variables with the Fisher’s Exact test. For individual variables, values during and after administration of the study drug were compared with base-line values by a mixed model analysis of variance on change from pre-infusion with the factors of time, treatment and time by treatment interaction. The different groups were compared by an analysis of covariance with treatment as a factor and the pre-infusion value as a covariate. Multiple comparisons between the treatment groups were performed with the Bonferroni correction. Differences were considered significant when P values were less than 0.05. All statistical analyses were performed with SAS version 8.
Cardialysis (Rotterdam, The Netherlands) was the core laboratory for angiographic and ECG analysis and the data management and coordinating centre. All listed authors (see appendix 1, online-only Data Supplement) participated in the study design, enrolment of patients, and/or data interpretation.
Results
Study population
A total of 47 patients were enrolled between December 2003 and March 2005. During this period, in November 2004, the Steering Committee temporarily suspended patient enrolment on the recommendation of the DSMB to permit a detailed analysis of a SAE described below. This SAE was adjudicated by the committee and individual review to be procedure and not drug related. The study was allowed to resume in January 2005. At this occasion the DSMB raised it’s concern about the critical elevations in SBP following IV HBOC-201 encountered in some patients and a protocol amendment instructing SBP management was issued. Of the 47 patients randomised, one patient withdrew consent before any study drug infusion and one patient did not receive any study medication; both patients were excluded from analysis. The remaining 45 patients concluded the planned 30-day follow-up. Analysis was by intention to treat, including one patient in whom the 30 g dose was inadvertently infused instead of the 15 g dose.
As shown in Table 1, treatment groups were equally matched with respect to age, weight, anginal status and the overall cardiovascular risk profile at screening.
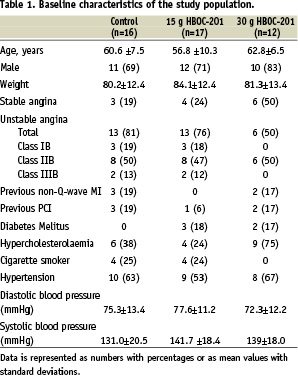
There were five diabetic patients in the HBOC-201 group and none in the control group.
Systemic hemodynamic effects
The most important haemodynamic effects of an IV infusion of HBOC-201 are summarised in Table 2.
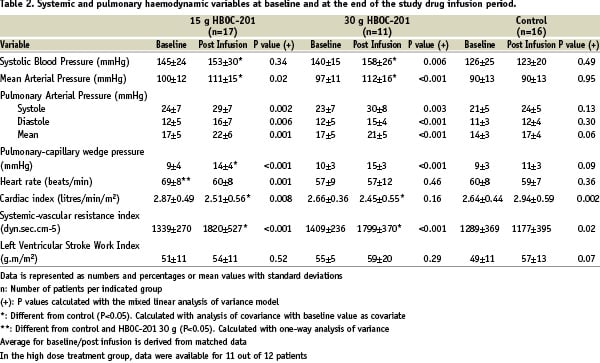
In both active treatment groups, there were significant increases in systemic arterial blood pressure (systolic, diastolic, or mean pressure) in conjunction with a significant reduction in cardiac index at 30 minutes after HBOC-201 infusion. The calculated systemic vascular resistance (SVR) (and pulmonary vascular resistance, PVR) was significantly increased in these patients. A dose relationship could not be established for these phenomena (Figure 1a-b-c-d).
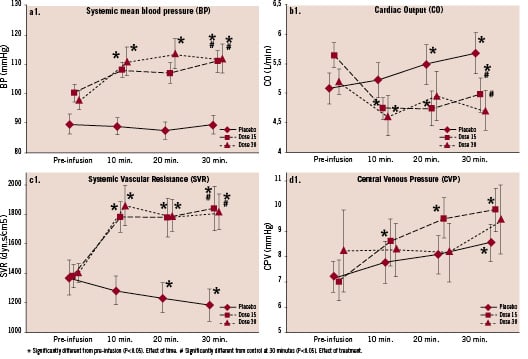
Figure 1.1 Relative change with respect to baseline values in systemic mean blood pressure (a), cardiac output (b), systemic vascular resistance (c) and central venous (right atrial) pressure (d). Effect of control or IV HBOC 15 g and 30 g on MBP (a1), SVR (b1), CO (c1) and CVD (d1).
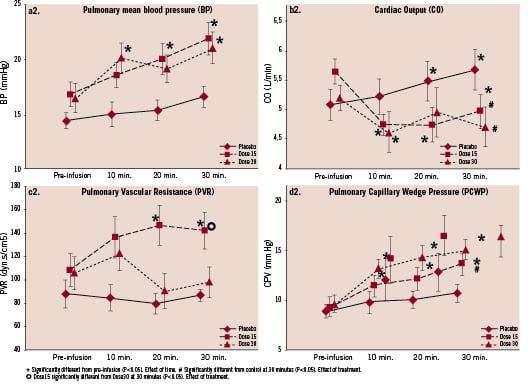
Figure 1.2 Relative change with respect to baseline values in pulmonary mean blood pressure (a), cardiac output (b), systemic vascular resistance (c) and pulmonary cardiac wedge pressure (d). Effect of control or IV HBOC 15 g and 30 g on PAP mean (1.2a), PVR (1.2b), CO (1.2c) and PCWP (1.2d). Measurements were made at baseline (pre-infusion) and at three different time-points during a 30 minute infusion period: 10’, 20’ and 30’=end of infusion. Values are shown as means ±SD for all patients.
Critical elevations in SBP following IV HBOC-201 administration, for the purpose of this study defined as a SBP >180 mmHg, was seen in 9/29 (31%) of the patients. Critical blood pressure elevations were reduced following the protocol amendment that instructed the use of appropriate antihypertensive treatment (IV nitroglycerin) when necessary; 7/20 (35%) patients before the amendment versus 2/9 (22%) patients post amendment. One patient was unresponsive to IV nitroglycerin and required nifedipine in order to control blood pressure. However, despite the reduction in absolute number of patients experiencing a clinically significant hypertensive episode, there was no difference between the amounts of nitroglycerin (NTG) used before and after the DSMB amendment instructing the use of NO donors to correct systolic blood pressure (a detailed description is provided in the online-only data supplement). At two hours post infusion (data not shown), any statistical difference in MAP remained between the active treatment groups and the control group. A significant decrease in heart rate was seen only in the HBOC-201 15 g group.
In all three groups the pulmonary capillary wedge pressure (PCWP) increased following infusion at 30 minutes; the increment was significantly greater in both HBOC-201 groups compared to control, (Table 2, Figure 1.2-d), never reaching the predefined critical level of 20 mmHg. There were no significant changes in calculated left ventricular stroke work index.
A significant decrease in mixed venous saturation (SVO2) was noticed following IV HBOC-201 (baseline: 77.4%±7.7%, 30 minutes: 70.7%±8.3%, p=0.002); in seven patients below the level of 65 %. However, the index of systemic oxygen consumption (VO2), assuming an arterial oxygen saturation (SaO2) of 97% in all patients, did not change from baseline (calculation, see online-only data supplement).
Coronary haemodynamic effects
The effects of IV HBOC-201 on the diameter of coronary arteries (reference vessel) and on flow velocity before and after IV adenosine administration are shown in Table 3.
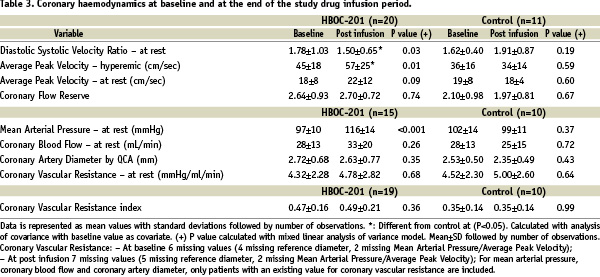
Intravenous administration of HBOC-201 caused no significant changes in the resting APV, coronary-artery diameter or coronary vascular resistance. The coronary blood flow velocity reserve tended to increase and this increase may be related to a significant augmentation in driving pressure. A detailed QCA analysis of the reference vessel did not show any angiographic coronary vasoconstriction brought about by the study drug (Table 1, online-only Data Supplement). Coronary flow studies were terminated early (n=31) after a futility analysis by the DSMB considering the presented data and the potential patient burden of this invasive procedure.
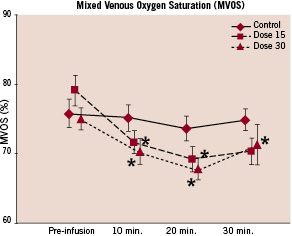
Figure 2. Relative change SVO2 with respect to baseline values. Measurements were made at baseline (pre-infusion) and at three different time-points during a 30 minute infusion period: 10’, 20’ and 30’=end of infusion. Values are shown as means±standard error.
Safety and tolerability
This study was aimed at providing as much safety information as possible about the IV administration of HBOC-201 in acute cardiology and PCI. The mean [±SD] amount of study drug solution infused in this study was: 238.1 (±34.2) ml for the 15 g HBOC-201group, 230.3 (±3.0) ml for the 30 g HBOC-201 group and 247.9 (±67.2) ml for the Voluven only group. One patient accidentally received two units of Voluven. In none of the patients did the study drug infusion have to be stopped for pre-defined safety reasons.
Patients were followed up for 30 days post PCI. During this period, no additional SAE occurred. The number of patients who experienced at least one adverse event was higher in the active treatment groups (75.9% pooled), as compared to the control group (62.5%) (Table 4), the difference is not significant statistically (p-value=0.49).
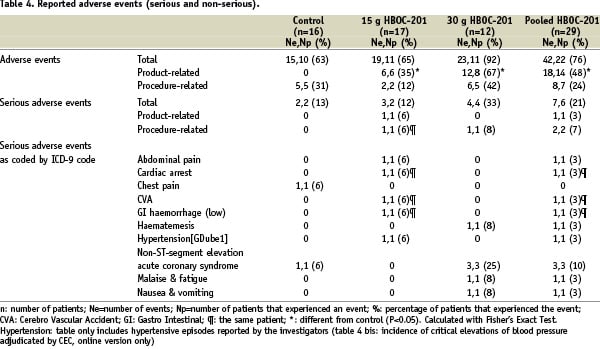
In total, eighteen adverse events were considered to be study drug related, one of which (a hypertensive episode) was serious. The difference in the number of events between the IV HBOC-201 treatment groups and the Voluven groups was mainly driven by the rise in liver (n=6) and/or pancreas enzymes (n=1) and the number of hypertensive episodes (n=10) (SBP>180 mmHg). In addition, one HBOC-201-treated patient experienced abdominal pain.
One patient suffered a periprocedural electromechanical dissociation (EMD), which followed a prolonged wedging of the guiding catheter during the PCI procedure. The patient required a prolonged resuscitation in the catheterisation laboratory. The clinical evolution was complicated by a watershed cerebral infarction and lower gastrointestinal bleeding. The patient experienced a full recovery. This incident was subject to detailed investigation by the DSMB supported by an independent neurologist while patient enrolment in the study was temporarily suspended. The EMD, reported as an SAE, was adjudicated to the procedure and not to the study drug; the neurological event reported as an SAE was putatively attributed to the period of hypotension experienced during the prolonged cardiac resuscitation of this patient. The cardiac arrest and “watershed infarction” were counted as one event.
No clinically significant changes were noted in haematological or chemical values following IV HBOC-201, except for the cardiac markers and liver transaminases. A significant rise in CK-MB levels >3 times ULN was documented in one patient, but this enzyme abnormality was not likely due to HBOC-201 treatment as the patient suffered procedure related serious complications and had a prolonged resuscitation period.
Through hospital stay, patients in both the HBOC-201 and control group had a similar incidence of increased liver alanine transaminase (31% vs 31%, respectively, NS); 10% of the patients in the active HBOC-201 group had elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT) or lactate hydrogenase (LDH) enzymes (>3 times ULN) compared with none in the control group (Table 5).
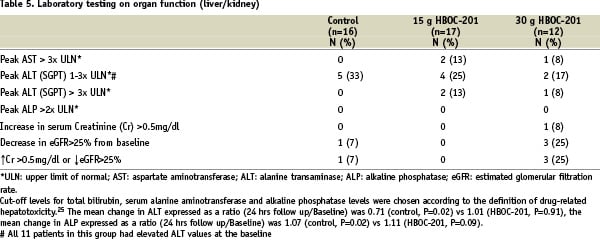
No patients had an abnormal total bilirubin (>3.0 mg/dl and > 100% increase) or Alkaline Phosphatase (>250 IU/L and > 100% increase) value, and no jaundice or hyper bilirubinaemia was reported. Two patients in the treatment group showed an increase in pancreatic enzymes (serum amylase >1.5 ULN). No effect of the drug was observed on the renal function (Table 5). Overall, there was a slight increase in plasma methaemoglobin level following IV HBOC-201 (average value pre-HBOC: 0.50% / 6-8 hours post-HBOC: 0.75%, P= 0.007 and post 18-24 hours 0.90%, P<0.001) with two patients above the cut-off level of 1.0% (data not shown).
The IV HBOC administration was associated with a statistically significant differential increase in inflammatory markers (IL-6, and CRP), measured at 18-24 hours after HBOC infusion, between the treatment arms and the control group, without any clear dose response relationship (Figure 3).
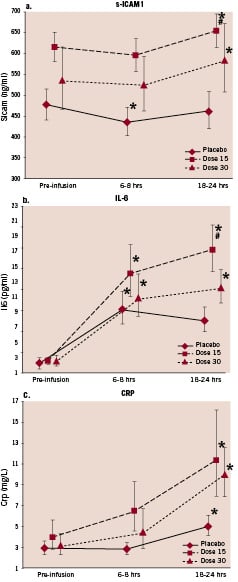
Figure 3. Effect of control or IV HBOC 15 g and 30 g on s-ICAM1 (a), IL-6 (b) and CRP (c). A dose relationship could not be established for any of these markers in the active IV HBOC-201 arms.Statistical analysis is performed on log-scale. For presentation, the geometric mean±the standard-error is given on the original scale. * Significantly different from pre-infusion (P<0.05). Effect of time.# Significantly different from control at 18-24 hours (P<0.05). Effect of treatment.
Discussion
We report on the first study in which HBOC-201 has been administered to patients with acute coronary syndromes undergoing PCI.
Several haemoglobin-based oxygen carriers are currently being studied in clinical trials for various indications. Most are derived from human or bovine blood and have been chemically modified, resulting in molecules that differ in size, molecular weight, oxygen affinity, viscosity, and oncotic activity. Every formulation should be considered a unique drug with its own physical characteristics, pattern of biological activity, and profile of adverse reactions.
HBOC-201 is a cell free, endotoxin free, glutaraldehyde cross-linked bovine polyhaemoglobin in solution with an average molecular weight of 250 kDa (molecular weight ranging 130-500 kDa) and a viscosity less than plasma (1.3 centipoise at 37°C). Only trace amounts (2%) of unmodified haemoglobin and stabilised tetramer (molecular weight 65 kDa) are detected. HBOC-201 has an oxygen dissociation curve that is right-shifted with a P50 of 40 mmHg, compared to 27 mmHg for native human haemoglobin. These features provide excellent oxygen-transport properties.
The pathobiological effects of cell-free plasma haemoglobin are a concern.12 Vascular homoeostasis is dependent on the compartmentalisation or physical separation of haemoglobin from the endothelium.13 However, unlike single haemoglobin molecules, polymerised-HBOCs like HBOC-201, that are mostly in the form of large soluble haemoglobin complexes (98% is > 130 kDa), are not expected to readily cross the intercellular endothelial junctions of blood vessels to exacerbate vasoconstrictive effects.
HBOC-201 is a colloid solution, and avoidance of circulatory overload is another important consideration. In our study population, a volume of up to 250 ml HBOC-201, equivalent to 30 g haemoglobin glutamer-250 bovine, was infused over a 30 minute time period. In none of the patients did the study drug infusion have to be discontinued for pre-defined safety reasons, such as an excessive increase in pulmonary wedge pressure.
IV HBOC-201 in this study population resulted in an increase in systolic blood pressure, a decrease in CO, and an increase in calculated SVR suggesting a vasoconstrictive effect. A critical elevation in SBP could be reversed by the intravenous administration of a nitric oxide donor, nitroglycerin, consistent with a putative role of nitric oxide scavenging in vasoregulation.14-17
The increase in SVR (afterload) in patients receiving HBOC-201 most likely contributed to the differential increase in PCWP between the control and treatment groups. The increase in preload observed after HBOC-201 must be interpreted as a normal physiological compensatory reaction to an increase in afterload (only observed in the HBOC-201 group) and to an increase in plasma volume expansion (induced in both groups). This increase in filling pressure does not reflect an intrinsic myocardial depressing effect of the compound nor a detrimental effect on the myocardial systolic function, since the Left Ventricular Stroke Work Index (LVSWI) remained unchanged regardless of the treatment received.
A decrease in mixed venous saturation was observed in both treatment arms and in some patients saturation went below 65% (Mean SVO2 at baseline 77.4, at 30 minutes 70.7, p=0.002). The most plausible explanation for this phenomenon is a reduction in resting cardiac output associated with study drug infusion; consequently the arterio-venous O2 difference would have to increase by lowering the mixed venous saturation. The metabolic demand of patients lying at rest on the cath lab table was probably unchanged and hence not a factor contributing to the fall in SvO2. There is no indication that IV HBOC-201 affected global oxygen consumption.
Our data clearly show that IV HBOC-201 had no effect on resting and hyperaemic coronary blood flow. This suggests that the autoregulatory mechanism of the coronary circulation was not adversely affected by the infusion of HBOC-201. In addition, there was no angiographic coronary vasoconstriction observed in the major epicardial vessel brought about by this drug.
This safety and feasibility study was designed to detect as many safety signals as possible; the DSMB was prospectively informed about the potential side effects of IV-haemoglobin solutions. The multitude of endpoints specifically scrutinised by either the investigators or the DSMB and CEC may have contributed to the apparently large number of adverse events reported.
Systemic removal of bioavailable nitric oxide has already been shown to contribute to clinical morbidities, including severe oesophageal spasm and dysphagia, abdominal pain and thrombosis.17-19 The low incidence of these nitric oxide-related clinical side effects in the present study, as compared to the literature, may be explained either by the lower dose of IV HBOC-201 used, by a concomitant use of nitric oxide donors and/or by the unique properties of the investigational drug.
A transient rise in concentrations of liver transaminases and/or pancreatic enzymes was seen in 10% of the patients following IV-HBOC-201. These patients were typically asymptomatic and without clinical sequelae during the 30 day follow-up. Since the liver is the normal Hb catabolic site, the absorption-distribution-metabolism and excretion (ADME) of HBOC-201 also involves the hepato-pancreatic systems, possibly inducing an upregulation of enzyme activity in response to an increased metabolic load. Elevations of transaminases and lipase have been observed in previous animal studies and clinical trials with HBOC-201. These enzyme elevations are generally not associated with hepatic or pancreatic dysfunction. The potential clinical importance of the increases in liver transaminases should be the subject of further investigation.
No adverse effect of the drug on renal function was observed. Nevertheless, given the recognised nitric oxide scavenging potential of the haemoglobin solutions, caution should be exercised when administering active HBOC-201 to patients with known renal dysfunction or in circumstances where the renal plasma flow is known to be reduced (i.e., NSAID use).
Nitric oxide reacts with oxyhaemoglobin to rapidly form the oxidation product, nitrate (NO3-), and methaemoglobin which is inactive.20 The comparatively slow reduction of methaemoglobin back to the active form makes the formation of methaemoglobin of potential clinical importance. In this study, the plasma level of methaemoglobin increased slightly following IV HBOC-201, remaining within the physiological range in most of the patients. When the circulating methaemoglobin values in both treatment doses were pooled, a significant difference was found between the pre-infusion value versus the 18-24 hour value (ratio 1.76, p=0.003). This difference is not considered clinically significant.
In our study, the circulatory levels of hs-CRP, IL-6 and s-ICAM in the whole population remained in the broad range of variability observed in patients with ACS undergoing PCI.21 The differential rise in circulatory levels of inflammatory markers following IV HBOC-201 compared to the control treatment is in accordance with previous observations indicating pro-inflammatory properties of plasma haemoglobin and heme.22 Heme stimulates the expression of the adhesion molecules ICAM-1 (intracellular adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1) and E-selectin on endothelial cells in vitro.23,24 The clinical significance and extent of our observations has to be established in further work.
Study limitations: These results reasonably apply to medium- to low-risk patients suffering CAD and cannot be extrapolated to patients with an evolving or recent transmural myocardial necrosis or haemodynamic instability because such patients were excluded from this study. IV HBOC-201 might provoke more pronounced systemic haemodynamic effects in patients with other cardiovascular conditions or different baseline characteristics. The favourable profile of HBOC-201 in this trial warrants additional animal studies and clinical trials, including in particular, studies in higher risk ACS (STEMI) patient populations. The current trial did not focus on myocardial oxygen consumption or tissue oxygenation during IV HBOC-201. Investigation of HBOC-201 oxygen transport properties and the potential for this therapeutic to preserve myocardial tissue oxygenation in humans is currently under way.
In conclusion, despite its known vasopressor effect, intravenous administration of HBOC-201 does not interfere with the autoregulation of coronary blood flow both at rest and after maximal hyperaemia. The safety profile of HBOC-201 in this study reflects many of the known side effects of haemoglobin based solutions. When clinically contextualised, the SAEs observed in the HBOC-201-treated patients arose from other factors and, with the exception of increased blood pressure, were not considered product related. However, some adverse events (AEs) of cell-free plasma haemoglobin observed in this study remain a concern and need further investigation. This work provides a first step towards exploring a new pharmacological strategy that could broaden the temporal window for PCI, particularly in patients suffering a STEMI. While we recognise that this small population study did not include STEMI patients, investigated only two doses of HBOC-201 and was not aimed at demonstrating efficacy or beneficial effects of this oxygen carrier during ischemia, the results are encouraging enough to pursue additional studies to gather that information.
Acknowledgements
The authors would like to thank Ms. Samantha Young for her assistance with the preparation of the manuscript.

