Abstract
Aims: Neovascularisation is mainly mediated by vascular endothelial growth factor (VEGF). Bevacizumab is a monoclonal antibody specific for VEGF. We assessed the safety of a bevacizumab-eluting stent, a dedicated stent for inhibition of plaque neovascularisation.
Methods and results: Patients with acute coronary syndromes and >2 significant coronary artery stenoses were included in the study. The culprit lesions should be successfully treated percutaneously. The targeted non-culprit lesions were <20 mm in length, producing significant stenosis (>50%). Local delivery of bevacizumab was accomplished via BiodivYsio stents which bear a phosphorylcholine coating that adsorbs the drug. Patients were discharged under aspirin and clopidogrel for six months. All patients were scheduled for clinical and angiographic follow-up at six months, and intravascular ultrasound (IVUS) imaging of the target vessel immediately after the procedure and at six months. Twenty consecutive patients were included. All stents were successfully delivered. During a follow-up period of 9.23±0.87 months there were no adverse cardiac events, including death, myocardial infarction and target vessel revascularisation. Angiographic and IVUS follow-up was performed in all patients. Acute, subacute or late thrombosis was not observed. Angiographic restenosis was not observed in any target vessel. In-stent late loss was 0.2±0.1 mm, and in-lesion late loss was 0.2±0.1 mm. Mean neointimal hyperplasia area in stented segments was 0.8±0.5 mm2 (12.4±7.7%) by IVUS.
Conclusions: The implantation of bevacizumab-eluting stents in human coronary arteries seems safe and elicits minimal neointimal hyperplasia. This new approach for high-risk plaque stabilisation through inhibition of plaque neovascularisation requires further investigation through placebo-controlled trials.
Introduction
Vasa vasorum are considered as the major source of nutrients to the vessel wall. Atheromatosis is associated with neovascularisation, as a dense net of vasa vasorum is developed in atheromatic arterial wall.1,2 Furthermore, an increased density of vasa vasorum in unstable atherosclerotic plaques promotes the vulnerability of the plaques.3 Neovascularisation is also related to the infiltration of inflammatory cells into the vessel wall.4,5
We have previously demonstrated the effects of removing vasa in experimental animal models.6,7 Medial necrosis and macrophage and smooth muscle cell infiltration occurred acutely even in morphologically intact endothelium, suggesting a role for adventitial vasa vasorum in the initial phases of the disease.
Recently, experimental studies have investigated the effect of agents,3,8,9 on neovascularisation. Moreover, new methods are emerging for the in vivo identification of increased neovascularisation in coronary lesions.10,11 However, these methods are still under development to specifically target angiogenesis in the setting of atherosclerosis.
None of the previous studies however have specifically targeted diagnostically or therapeutically the vascular endothelial growth factor (VEGF). It is known that in the process of neovascularisation the VEGF, a potent regulator of physiological and pathological angiogenesis,12,13 is considered as the key regulator. Bevacizumab, an antibody specific for VEGF, has recently been applied in the clinical field for the treatment of carcinoma.14
We hypothesised that local delivery of bevacizumab by stent would inhibit the development of neovascularisation in human coronary arteries. We have previously shown that bevacizumab-eluting stent implantation in rabbit iliac arteries is feasible and safe15. Moreover, neovascularisation is inhibited with an associated favourable effect on neointimal hyperplasia without affecting the endothelialisation rate, the fibrin deposition score and the inflammatory response.16 In this first-in-man (FIM) study we assessed the feasibility of local delivery of bevacizumab by a phosphorylcholine-coated stent in human coronary plaques. Clinical, angiographic and intravascular ultrasound (IVUS) follow-up examinations were scheduled for the evaluation of the safety of the device.
Methods
Study population
A pre-specified population of 20 patients with acute coronary syndromes (ACS) and > 2 angiographically significant coronary artery stenoses were included in the study. Non-ST elevation myocardial infarction was diagnosed in 15 patients with mean troponin I level of 2.1 ng/ml, and five patients were diagnosed with a ST elevation myocardial infarction, (STEMI) with a mean troponin I level of 22.3 ng/ml. The culprit lesions should be successfully treated percutaneously at the operator’s discretion. For the non-culprit lesion the angiographic inclusion criteria for bevacizumab-eluting stent implantation were: significant lesion, < 20 mm in length in a major native coronary artery; and a proximal reference vessel diameter > 2.5 mm. The Institutional Ethics Committee approved the study protocol, and each patient provided written, informed consent.
Loading bevacizumab stents
For local delivery of bevacizumab, we used the BiodivYsio stent delivery system (Biocompatibles Ltd., London, United Kingdom). Briefly, the stent is a laser cut, 316L stainless steel balloon-expandable stent coated with phosphorylcholine (PC). The biocompatible PC coating constitutes a double layer of synthetic PC head-group coating that absorbs a drug via a “sponge-like” mechanism.17-19
The method of impregnating the PC coating involved three steps. The stent is first immersed into a solution of 4 ml bevacizumab (Avastin, 25 mg/ml, Roche) for five minutes. The stent is then removed from the solution and after allowing it to dry for one minute, a second step by 10 µl of the same solution was pipetted onto the stent. The stent was again allowed to air-dry for one minute. This process was repeated, with additional five minutes of air-drying. The total preparation time for loading bevacizumab on the stent was approximately 12 minutes. Animal studies have revealed the safety of this approach. Laboratory testing has demonstrated that approximately 80% of the drug is eluted in the first 48 hours.
Procedure of implantation
The procedure of stent implantation was performed according to the standard percutaneous coronary interventions protocol. After engagement of the guiding catheter to the coronary ostium, the bevacizumab-eluting stent was then advanced and implanted in the stenotic segment of the coronary artery. After predilatation of the target lesion, the stents were deployed with high pressure. Mean stent diameter was 2.82±0.11 mm, and mean inflation pressure was15.2±atm. A final angiogram was performed to confirm the optimal expansion of the stents. All patients received aspirin (325 mg/d, indefinitely), which was started at least 12 hours before the procedure, and clopidogrel (300 mg immediately after stent implantation and 75 mg/d for six months).
Quantitative measurements
Quantitative coronary angiography (QCA) and IVUS imaging were performed immediately after the procedure and at 6-month follow-up in all patients after a bolus infusion of intracoronary nitrates. IVUS images were acquired using motorised pull-back at a constant speed of 0.5 mm/s. Quantitative angiographic and IVUS analyses were performed by investigators (MV, ES), blinded to the clinical characteristics and the type of the implanted stent. The mean total vessel area (VA), mean stent area (SA), and mean minimal lumen area (MLA) were measured. Mean neointimal hyperplasia area (NIHA) was derived by SA-LA at follow-up examination. Percentage of NIHA and was then calculated as NIHA/SAX100.
Statistical analysis
Continuous variables are expressed as mean±SD. Comparisons between post-intervention and follow-up measurements were performed with a 2-tailed paired t test. P<0.05 was considered statistically significant.
Results
All patients were admitted to the hospital due to acute coronary syndrome (ACS) and the procedure was performed within 24 hours after admission. The demographics characteristics, risk factors clinical features of the studied population are shown in Table 1.

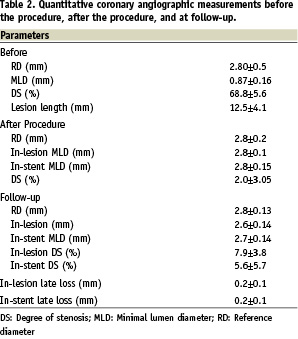
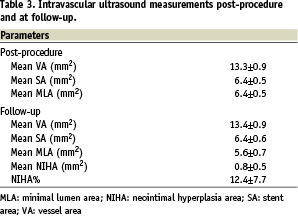
All stents were implanted successfully (mean stent length 13.5±4.1 mm), and all patients were discharged without any complication. Creatine kinase and creatine kinase-MB levels, sampled at six and 18 hours after the procedure, were within the normal range in all patients. Angiographic and IVUS data are presented in Tables 2 and 3, respectively.
During the clinical follow-up period of 9.23±0.87 months adverse cardiac events, including death, myocardial infarction or target vessel revascularisation were not observed. Angiographic and IVUS follow-up did not reveal any restenosis (50% vessel narrowing by QCA or IVUS). The distribution (%) of vessel and lumen areas post-procedure and at follow-up are demonstrated in Figure 1.
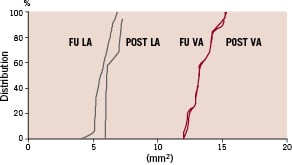
Figure 1. Left, Cumulative distribution curves of lumen (LA) and vessel (VA) areas post (POST) bevacizumab-eluting stent implantation and at follow-up (FU).
In four patients, the neointimal hyperplasia area (%) was more than 15% (Figure 2).
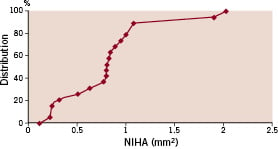
Figure 2. Cumulative distribution curves of neointimal hyperplasia area (NIHA) in bevacizumab-eluting stent treated patients at follow-up.
Stent malapposition was not observed in any patient. There were no repeat revascularisations, stent thromboses, or major clinical events (cerebrovascular accident, myocardial infarction, or death).
Discussion
This is the first human experience with the implantation of bevacizumab-eluting stent. The results of the present study, including clinical, angiographic and intravascular ultrasound examination at follow-up demonstrated that bevacizumab-eluting stent implantation, dedicated for atheromatous plaque neovascularisation inhibition, is feasible and seems to be safe.Plaque neovascularisation has been recently identified as a characteristic of high-risk plaques prone to rupture5,20. A correlation between the extent of atherosclerosis and plaque neovascularisation in human pathological samples,5 and in the coronary arteries of hypercholesterolaemic primates has been observed.21 In specimens with chronic inflammatory cell infiltration by macrophages and lymphocytes, an increased number of micro-vessels are observed.2 The VEGF, a potent regulator of physiological and pathological angiogenesis, plays a major role in plaque neovascularisation and therefore is a potential therapeutic target for the inhibition of plaque neovascularisation.1 Recently, several agents, such as simvastatin8, antioxidants9, and vitamins C and E3, have been used for the inhibition of plaque neovascularisation. However, none of these agents targets specifically at VEGF, which has been recognised as a major regulator for the development of microvessels at the atheromatic arterial wall.
Bevacizumab, a recombinant humanised monoclonal antibody against VEGF, has been applied in oncology as a potent anti-angiogenic agent. We used the BiodivYsio stent for local delivery of bevacizumab at the target lesion, as the specific stent has been used successfully in previous studies delivering possible anti-hyperplastic agents. After the experimental application16 of the study, we proceeded to the clinical application of this stent in 20 patients. In this study we focussed on patients with acute coronary syndromes, as several studies have shown that there is increased inflammatory activity in both culprit and non-culprit lesions. The stent was implanted in non-culprit lesions, as these lesions are prone to rupture and produce future adverse events.1
In this study the feasibility and safety, by using angiographic and IVUS data, was demonstrated by the favourable clinical outcome and mainly by the angiographic and IVUS data. Although the mean neointimal hyperplasia area (%) is greater than those of other drug eluting stents, such as the sirolimus22 and paclitaxel-eluting stents23, it was less than that of bare metal stents. Moreover, the inhibition of neointimal area was uniformly distributed along the stent, indicating the homogeneous longitudinal diffusion pattern of bevacizumab from the stent. These findings showed that bevacizumab-eluting stent implantation was not accompanied by adverse events, and may have also anti-hyperplastic effects, although this was beyond the scope of this study. Despite the lack of the in vitro kinetics of bevacizumab, the reduced VEGF expression compared to the control group in the arterial wall of rabbits is indicative of the effect of bevacizumab.16
Double antiplatelet treatment was administered for a period of six months. In previous studies with phosphorylcholine-coated stents, patients did not receive prolonged antiplatelet therapy.24-27 Despite these clinical data and the encouraging experimental results with bevacizumab-eluting stent16, combined antiplatelet treatment was administered for a prolonged period to avoid any thrombotic events. The presented clinical follow-up results showed, however, that even after the discontinuation of clopidogrel at six months, late thrombosis was not observed up to nine months. This can be explained by the neutral effect on endothelialisation compared to phosphorylcholine-coated stents.16 Thus, in future studies an even shorter period of dual antiplatelet treatment is justified to be investigated.
Clinical implications
The results of the present study have important pathophysiological and clinical implications in our view. The minimal neointimal hyperplasia, observed by IVUS, confirms the experimental results, in which the mean neointimal thickness and area were less in the bevacizumab group compared to the control group.16 In this study, patients with acute coronary syndromes were included in which several studies revealed a widespread vulnerability.28,29 The reduced neointimal hyperplasia may be correlated to the anti-angiogenic effect of bevacizumab, as vulnerable plaques are associated with increased neovascularisation.5 Although there was no direct evaluation of the local inflammatory activation in the treated lesions of the present study, a possible correlation between plaque neovascularisation and intimal hyperplasia needs to be further investigated.
Moreover, previous studies showed that by inhibition of plaque neovascularisation a reduction of macrophage accumulation is accomplished.4 Bevacizumab may reduce the hyperplastic response post-stent implantation through an anti-inflammatory effect, although in rabbit iliac arteries there was no difference in the inflammatory score between bevacizumab-eluting stents and phosphorylcholine-coated stents.16
Another challenge in the clinical field is the assessment of inhibition of plaque neovascularisation in the target lesions. Currently, there is no validated method for the in vivo quantification of microvessels in human coronary arteries. New techniques are being developed by IVUS for the imaging of vasa vasorum. A possible combination of diagnostic and therapeutic tools can prove the concept in vivo in humans. However, a study evaluating the safety and effectiveness of this approach, in terms of clinical events and angiographic parameters, needs to be performed.
Study limitations
First, the small population included in this first-in-man study without a control group did not allow for conclusions regarding the efficacy of the stent compared to bare or drug eluting stents. Although adverse events were not encountered, and clear conclusions cannot be drawn regarding the safety of this approach, there were no late thrombotic events despite the discontinuation of double antiplatelet treatment at six months. The lack of any thrombotic event after the cessation of the combined antiplatelet treatment is indicative for the safety. However, in the era of drug-eluting long-term clinical follow-up is required to evaluate the safety of the devices. Second, patients had rather low risk angiographic characteristics, although we included patients with acute coronary syndromes, which are accompanied by higher risk for future events. Third, the results of the present study are limited to nine months, and may not predict subsequent findings identified in this data set. Finally, we did not have a control group to evaluate the efficacy of this approach, as a specific treatment for local passivation of high-risk plaques has not been established.
Conclusions
Bevacizumab-eluting stents seem to be safe for the treatment of non-culprit de novo lesions in patients suffering form acute coronary syndromes. The results of the present study provide new insights in the stabilisation strategies of high-risk plaques. These findings warrant further confirmation by large, placebo-controlled, multicentre trials.

