Abstract
Aims: We report the acute and 30 day results of the OPEN I study, a multicentre prospective single arm study evaluating the safety and feasibility of the Stentys™ bifurcation stent.
Methods and results: The Stentys™ stent is a provisional, self-expanding nitinol drug eluting or bare metal stent with small interconnections that can be disconnected by balloon angioplasty to provide access to the side-branch and full ostium coverage. Forty patients with de novo coronary bifurcation lesions were enrolled to be clinically followed-up over four years. In addition to angiographic QCA evaluation, documentary IVUS and/or OCT were used in all cases to assess the stent’s deployment. The patient population consisted of 85% males with an average age of 62 years. Almost half had previous PCI, 31% previous MI and 5% previous CABG. The majority of lesions (80%) involved the LAD-D, 42% of the patients had disease affecting the side-branch, with all three arms diseased in 24% of the cases. The average lesion length in the main branch was 12.95±3.63 mm with a bifurcation angle of 55° (range 30°-80°). Procedural success was achieved in 39/40 cases (95.5%) due to inability to track the stent in one patient with an extremely tortuous vessel. In total 6 (15%) paclitaxel eluting and 33 (85%) BMS Stentys™ stents were successfully implanted, and simple disconnection of the stent mesh overlying the SB ostium was achieved in 37/39 cases (94.9%); in two cases, disconnection was not attempted. The MACE at 30 days was 5.1% as a result of one non
Q-wave MI following the procedure and one ischemia-driven revascularisation six days after the procedure.
Conclusions: This first-in-man (FIM) study demonstrates that the Stentys™ stent is safe and feasible resulting in an excellent procedural success rate and a low MACE rate. The struts can be easily and safely disconnected to perform provisional stenting.
Introduction
Bifurcation disease accounts for up to 20% of all coronary disease treated by percutaneous coronary intervention (PCI)1. However, the lower procedural success – especially as the result of abrupt side-branch (SB) closure with single stent strategies together with the early thrombosis and high restenosis associated with the complex refashioning of the carina with two stent techniques – remains a common reason for referral to coronary artery bypass grafting2. Current coronary stents were designed for straight lesions, optimised to provide adequate scaffolding (coverage and radial strength) and ease of deliverability. In straight lesions, these stents have been shown to provide good acute and long-standing results3. An attractive strategy for managing bifurcation must take this into account, but at the same time provide easy access to the SB and adequate coverage of the ostia should the need arise to provisionally stent the SB. This basic concept is realised in the Stentys™ stent (Stentys SAS, France) (Figure 1).
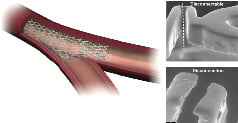
Figure 1. Showing the Stentys™ stents struts disconnected with an angioplasty balloon. Electron microscopy (right panel) shows clean disconnection.
We report the 30 day outcome of the Stentys™ OPEN I study: (Stentys Coronary Bifurcation Stent System fOr the PErcutaNeous treatment of de novo lesions in native bifurcated coronary arteries), a multicentre, prospective, single-arm study evaluating the safety and feasibility of the Stentys™ stent to treat de novo bifurcation lesions within the coronary vasculature.
Method
The Stentys™ stent
The Stentys™ coronary stent is a provisional, self-expanding nitinol drug eluting or bare metal stent designed to treat bifurcations with significant side-branches4. The novelty is that the stent shape is adapted to the bifurcation anatomy after implantation. This is due to the stents’ unique self-expanding nitinol Z-shaped mesh that is linked by small interconnections. It offers the stent’s struts the ability to be disconnected by an angioplasty balloon to create the SB access so to achieve optimal ostial scaffolding. The Stentys™ stent struts have this property all around and along (except for the most proximal and distal segments of 2.5 mm each) so that the initial positioning is irrelevant, solving a problem often encountered in the more cumbersome dedicated bifurcation stent designs (Figure 2).
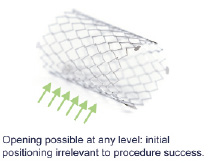
Figure 2. Stent positioning.
The stent is 18 mm long and has the potential to expand to a maximum of 7.0 mm diameter at the carina. It means that it is ideally suited for the most commonly found bifurcations having diameters of 3.5 mm to 4.5 mm in the proximal main branch (MB) and 2.5 mm to 3.0 mm in the SB. A foreshortening of less than 10% should be expected.
In the drug eluting form, paclitaxel (0.8 µg/mm2 of stent) is incorporated in ProTeqtor®, a durable polymer matrix of polysulfone (PSU) and uses soluble polyvinylpyrrolidone (PVP) as the excipient. A 7 Fr GC-compatible rapid-exchange delivery system delivers the stent in position over a single 0.014” guidewire in the main vessel by withdrawing a retractable sheath. A second 0.014” guidewire is used to cross the ostium allowing access for a standard angioplasty balloon into the SB to disconnect the stent struts and anatomically reconstruct the bifurcation shape. Maximum ostial coverage is best achieved with bifurcating angulations of 30° to 70° by opening of the cell closest to the carina. The delivery system has a marker on the end of the sheath and on the stent stopper to facilitate easy deployment. Following deployment, a commercially available workhorse stent can be effectively deployed in the SB to treat any residual disease without leaving a gap.
Stentys™ stent OPEN-I protocol
Study design
This was a multicentre, prospective, non-randomised single arm study. The primary objective was to assess the safety and feasibility of the Stentys™ stent in de novo native coronary bifurcated lesions. A total of 40 patients were enrolled between September 2007 and September 2008 in nine European clinical sites.
Patient population
Patients were enrolled if they had (un)stable angina or any objective evidence of ischaemia in the territory of the bifurcation lesion. In addition, the bifurcation had to comply with 3.5 mm to 4.5 mm diameters in the proximal MB and 2.5 mm to 3.0 mm in the SB, together with having a bifurcation angle of 30° to 70°. The lesions lengths were typically ≤15 mm with stenoses >50% and <99%. Diabetics and patients with left main stem or chronically occluded or heavily calcified vessels were excluded. The lesions were treated with a single Stentys™ stent. Three centers were selected to test the DES version. The bare metal version was to be placed in all other centres and patients. If deemed necessary, a work-horse drug eluting or bare metal stent was deployed in the SB. All patients are required to be clinically followed for up to four years. The anti-platelet regimen consisted of aspirin combined with clopidogrel and/or ticlopidine per institutional standards of practice.
Study endpoints
The primary endpoint was the procedural success which was defined as technical and angiographic success in the absence of a major adverse cardiac event (MACE) at hospital discharge. Angiographic success was achieved if there was <30% residual stenosis in MB and SB with TIMI 3 flow in both vessels post-procedure. MACE included cardiac death, stroke, myocardial infarction (MI), coronary artery bypass graft and target lesion revascularisation (TLR). A MI was said to occur if there were new abnormal Q-waves in accordance with the Minnesota Code for pathologic Q-wave or if the creatinine kinase (CK) was twice the upper limit of normal and there was a rise in the level of MB iso-enzyme of CK (CK-MB). TLR was defined as a repeat treatment of a lesion located within the index coronary artery segment. TVR was defined as a revascularisation of any segment of the index coronary artery.
The secondary endpoints included clinical, safety and angiographic parameters. Clinically, the MACE and anginal status were assessed at one month together with justified and non-justified revascularisation. Safety parameters included major bleeding and major vascular complications. Angiographic success was determined by off-line pre- and post- procedural quantitative coronary angiography (QCA) performed using the dedicated CAAS 5 bifurcation software (Pie Medical, Maastricht, Netherlands)5 at the core laboratory facility of Cardialysis, Rotterdam, The Netherlands. IVUS and/or OCT of MB and SB was mandatory before stent implantation for sizing purposes, also after disconnection of the stent following implantation, and in case of SB stenting.
Results
Demographics
Most of the patients (88%) presented with stable angina symptoms and their demographics are entailed in Table 1.
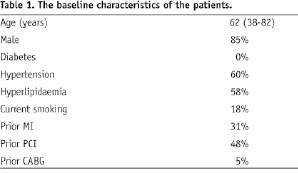
Patients were typically male (85%) and had an average age of 62 years. Almost half had previous PCI, 31% previous MI and only 5% previous CABG. All patients were admitted on aspirin and were preloaded with clopidogrel.
Lesion characteristics
The location of the disease is illustrated in Figure 3; the majority (80%), involving the left anterior descending-diagonal arteries (LAD-D).
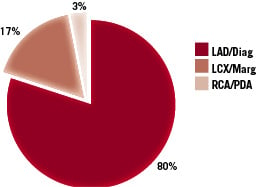
Figure 3. The location of the diseased bifurcations.
The major (94%) lesion type was ACC/AHA type B (78% B2) with 6% type C. According to the Medina classification for bifurcations, 16 patients (42%) had disease affecting the SB, and in nine patients (24%) disease affected all three arms of the vessel (Figure 4).
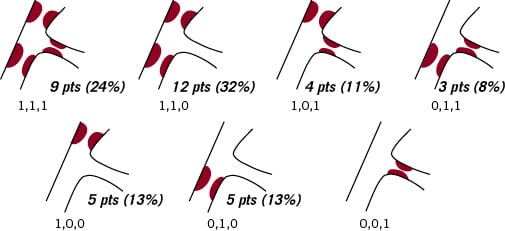
Figure 4. The Medina classification of the bifurcations.
No patients were treated for disease contained solely in the SB, implying that all had disease in the MB. Initial evaluation of the bifurcation revealed that the average lesion length in the MB was 12.95±3.63 mm. The average bifurcation angulation (between the SB and MB) was 55° and ranged 30°-80°.
Primary endpoint: procedural success and MACE
Amongst the study population of 40 patients there was only one case where stenting was not possible due to the fact that the device could not be tracked in a very tortuous circumflex artery bifurcation. The resulting procedural success rate was thus 95.5%. Prior to deployment, lesions were prepared by predilating the MB only (22 cases, 55%), the SB only (one case, 3%), SB and MB (13 cases, 33%) and with simultaneous kissing balloons (SKB) in three cases (8%). In one case (3%), no predilation was performed. Of the Stentys™ stents implanted, six (15%) were paclitaxel eluting with the remaining 33 (85%) being the Stentys™ bare metal stent (BMS).
Disconnection of the strut overlying the ostium of the SB was successfully achieved in 37/39 cases (94.9%). In two cases, disconnection was not attempted; indeed the Stentys™ Stent deployed to cover a lesion in the distal MB was malpositioned distally and the non-disconnectable proximal end of the stent was covering the SB ostium. Following Stentys™ stent deployment in the MB, it was deemed necessary to stent the SB in one third of the cases (13/39): eight cases with a drug eluting stent and five cases with a bare metal stent. All stents were placed using the T–stent approach (no culotte or mini-crush technique). These stents were optimised using SKB which was used overall in 14/39 cases (52%). In three cases (11%) no post-dilation was performed, and in the remaining 21 cases (45%) either the MB (proximal and/or distal part) and/or SB were postdilated. In some cases where OCT was performed, non-quantitative analysis showed that there was complete apposition of the stent struts to the vessel wall. IVUS analysis will be done when all six months data are available.
It was found necessary to deploy additional stents in the main vessel to cover tandem lesions or dissections as illustrated in Figure 5.
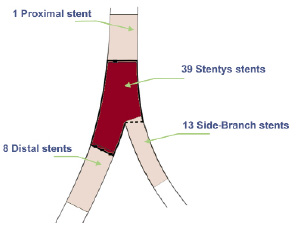
Figure 5. Additional stent placement following Stentys™ stent implantation in the MB (N=39).
There were two MACE at 30 days as adjudicated by the Clinical Events Committee (CEC) – one in-hospital MACE with a non Q-wave MI following the procedure (a temporary rise in CK-MB without sequelae), and one other patient developed chest pain six days after the procedure and underwent revascularisation of the ostium of the SB in another hospital (Table 3).

Quantitative coronary angiography (QCA), pre- and post- stent implantation
The QCA analysis pre- and post- stent implantation was obtained for 38 patients, since in one case no stent was deployed and in another case no post procedure analysis could be undertaken because of significant vessel overlap obscuring the radiographic images analysed. Taken as a whole, including all combinations of Medina classifications results in average numbers for all proximal MBs, distal MBs and SBs as displayed in Table 2.
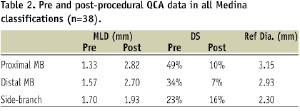
To assess the Stentys™ stent performance in the diseased MB, subgroup analysis of the 12 patients with disease only in proximal and distal MB (1,1,0) demonstrated an acute gain of 1.74 mm (pre=1.19±0.40 mm, post=2.93±0.41 mm) and 1.17 mm (pre=1.64±0.50 mm, post=2.81±0.41 mm) respectively (all differences of the means). In the five cases where only the proximal MB was diseased, the acute gain was 1.86 mm (pre=1.14±0.40 mm, post=3.00±0.14 mm).
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT)
Preliminary IVUS and OCT revealed excellent apposition of the stent to the vascular wall as a result of the Stentys™ stent self-expanding nitinol properties. Moreover, in cases where the struts were disconnected there was appropriate ostium coverage (Figure 6).
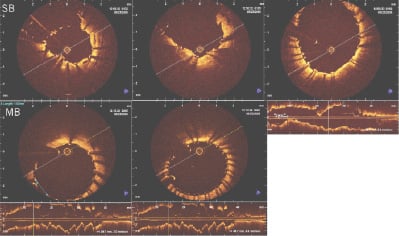
Figure 6. In vivo OCT after bifurcation stenting showing adequate apposition and carina coverage following T- stenting of MB and SB.
Discussion
This study demonstrates that the Stentys™ stent can be safely deployed with excellent procedural success. In addition, it provides side-branch access and allows provisional treatment of the branching vessels. Unlike some dedicated bifurcation stents, it employs a single wire usage and easy entry of side-branches and so avoids wire wrap. As one of the very few available self-expanding coronary stents, its properties mean that the Stentys™ stent readily conforms to the complex peri-carinal vessel anatomy and shows lack of stent strut deformation, which can be clearly visualised by IVUS and StentBoost Subtract6 as illustrated in Figures 7 and 8.
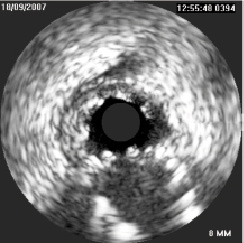
Figure 7. IVUS pullback from 1st diagonal at the carina.
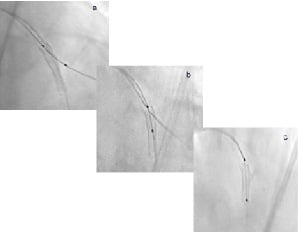
Figure 8. StentBoost Subtract images of deployed stent before (a), during (b) and after (c) disconnection.
Also, the self-expanding stent appears to have adequate radial strength as shown by QCA data related to residual stenosis.
Scaffolding the SB ostium is achieved after disconnection of the struts as they extend into the SB. The results show that the device is effective over a range of bifurcating angulations and the simple struts disconnections mean that a standard workhorse stent can be easily tracked and positioned to be deployed in the SB for optimisation with SKB. The stent also had a relatively low MACE rate (5.1%) at 30 days due to a single periprocedural NSTEMI and one TLR with a Stentys™ BMS.
The best approach to manage a bifurcation to achieve optimal procedural outcomes and, more importantly, long-term success with low restenosis rates and low MACE rates is still heavily debated7. Simple stent strategies that allow for optional provisional stenting remain an attractive option8,9. The Nordic Bifurcation Study10 and a large observational registry11 have shown that complex stenting of bifurcations does not offer advantages over stenting the main vessel with optional stenting of the SB. In the CACTUS study12, the crush technique was compared with provisional T-stenting. Crossover from a single stent approach happened in 31% of the cases. This study confirmed findings from other studies that failed to show superiority of a strategy of complex double-vessel stenting. Moreover, fractional flow reserve (FFR) assessment of the “jailed side-branch” has commonly failed to show functional significance (FFR <0.75), even in cases where there is a >75% stenosis at the ostium of the side branch13 and follow-up data at nine months on reopening jailed side branches did not influence MACE14. In addition, routine T-stenting with sirolimus eluting stents did not improve the angiographic result in bifurcations as compared with stenting of the MB followed by SKB dilatation and provisional side-branch stenting15 and may support the Stentys™ stent approach.
Limitations
This was a first-in-man (FIM) study in a small number of patients. The design was non-randomised and only the 30 days results were reported. Although the study results give a clear indication of the feasibility and short term safety of the stent, longer follow-up data and randomised study designs will lead to more definite conclusions.
Conclusions
This FIM study on the Stentys™ stent demonstrates that the device is safe and feasible, resulting in an excellent procedural success in 39/40 patients and a low MACE rate (one in-hospital enzyme rise and one revascularisation six days after the procedure). In all cases where this was attempted, the stent’s struts were easily and safely disconnected to perform provisional stenting as was deemed appropriate by the operator.

