Abstract
Aims: To evaluate the safety and long-term efficacy of a true dedicated bifurcation bare metal stent (DBS) for the treatment of bifurcation coronary artery lesions.
Methods and results: Thirty-four patients were enrolled in this prospective multicentre study. The majority of culprit lesions were located on the left anterior descending artery/diagonal bifurcation (n=19) followed by the distal protected left main (n=7), the left circumflex artery/obtuse marginal (n=4) and the distal right coronary artery/posterior descending artery (n=4). Successful delivery of the DBS stent at the bifurcation site was achieved in 32 patients (94%). Angiographic follow-up at six months was complete in 29 patients (91%). Clinical follow-up was achieved at five years in all DBS patients. There were no cardiac deaths or stent thrombosis. At six months, the MACE rate was 6/32 (19%) and the total binary restenosis rate was 10/29 (34%). MACE at 5 years consisted only in target vessel revascularisation and occurred in eight patients (25%).
Conclusions: The DBS bare metal true bifurcated stent can be delivered successfully and safely in selected bifurcated lesions and has demonstrated long-term efficacy in most patients.
Introduction
Percutaneous transluminal coronary angioplasty (PTCA) is the first revascularisation option for treatment of most patients with coronary artery disease. Clinical results have improved with the development of stents, reducing the rate of major adverse cardiac events (MACE)1,2. Drug eluting stents (DES) have significantly reduced the restenosis process3,4. As expected, treatment of lesions located at the bifurcation of two vessels is more difficult and is associated with a higher rate of restenosis than treatment within a straightforward lesion. Provisional stenting technique and at the opposite the “crush technique” have not solved the problem in this setting, even in the DES era. Treatment of these complex lesions is technically challenging and associated with higher complication rates5-18. Therefore, the treatment of bifurcation lesions has not yet reached a technical consensus.
In order to avoid the difficulties encountered in implanting stents in both branches of the bifurcation and to make the side branch (SB) access through the main stent easier, a new generation of dedicated stents has been designed and tested19,25. The DBS stent is an original dedicated bifurcated bare metal stent with two distal legs, designed for the treatment of de novo bifurcation coronary artery lesions, such as distal left main artery (LM) stenosis. The purpose of the present first-in-human (FIH) pilot study was to assess the technical performance of the DBS stent in selected patients, in terms of feasibility and long-term safety.
Methods
Study design and participating centres
The FIH DBS study is a pilot multicentre, prospective, single-arm registry trial, evaluating the safety and efficacy of the DBS stent in lesions involving bifurcations of native coronary arteries. Four centres in France have participated in the trial (see Appendix). The study protocol was approved by the local ethics committee and the institutional review board of each participating centre. Written informed consent was obtained from all patients.
Patient selection
All the patients included in the study suffered from coronary disease due to native coronary artery lesions. They presented with stable angina according to the Canadian Cardiovascular Society or unstable angina according to Braunwald classification 1 to 2 A, B and all demonstrated objective evidence of myocardial ischemia attributable to the target lesion26,27. In accordance with the device characteristics, angiographic inclusion criteria were a significant (reduction in diameter > 50%) bifurcation stenosis with a maximum lesion length of 14 mm on the main branch (MB) and 8 mm on the SB. The reference diameter (RD) of the main target vessel had to range between 3.0 and 3.5 mm, proximal to the bifurcation and the SB RD had to range between 2.75 and 3.0 mm. During the study time (between April 2000 and July 2001), 4,315 patients have been treated by PTCA in the four participating centres on 6,373 coronary lesions. Among these lesions, 1,591 lesions involved at least one bifurcation site (25%), 1,132 (18%) were treated as a simple lesion and 459 (7%) were treated as true bifurcations. According to inclusion criteria, 34 patients with 34 bifurcated lesions were included in this trial.
Patients who had suffered from myocardial infarction within five days prior to PTCA and presenting with CPK elevated three times above the normal values, or who presented with unstable angina according to Braunwald Classification type C, and patients with any contraindication to aspirin, ticlopidine, clopidogrel or heparin therapy were excluded from this study.
Angiographic criteria for exclusion were the presence of thrombus in the bifurcation lesion, a bifurcation lesion in a non-protected LM, and left ventricular ejection fraction of < 35%.
Stent design and delivery system
The DBS coronary stent (Cordis Corp, a Johnson & Johnson Company, Miami, FL, USA) is a slotted 316L stainless steel tubular balloon expandable stent with two asymmetric legs. The stent design incorporates two stents, laser cut from seamless tubing, electropolished and welded to form the final assembly of a bifurcated stent. Strut thickness is 0.0047”/0.12 mm. The MB stent is 15 mm long with two parts of an equal length of 7.5 mm: a proximal part (segment I) with a side aperture and a distal part (segment II). The SB (segment III) stent length is 8 mm, with a tapered shape proximal extremity in front of the side aperture of the MB. This shape provides side branch scaffolding (Figure 1A). SB was defined as diagonal branch for bifurcation lesions involving left anterior descending (LAD) and diagonal arteries, obtuse marginal branch for left circumflex (LCX) bifurcations, posterior left ventricular for right coronary artery (RCA) distal lesions, LAD for protected distal LM lesions. The main and side branches are joined by a short flexible bridge. For the present study, two balloon sizes were available. The larger size was 3.5 mm for the MB and 3.0 mm for the SB. The smaller size was 3.25 mm for the MB and 2.75 mm for the SB.
The DBS Stent is pre-mounted on two 18 mm long independent RaptorTM semi-compliant balloons over the wire delivery system (Cordis Corp, a Johnson & Johnson Company, Miami, FL, USA). Both balloons are side-by-side in the proximal part of the stent and are separated distally, as each is engaged in one leg of the bifurcated stent. Each balloon catheter has two platinum iridium radio-opaque marker bands. In the MB, balloon marker bands indicate the balloon working length, whereas in the SB balloon marker bands indicate the distal end and the intersecting point of both branches, i.e., the carina. The delivery system has a usable length of 145 cm. The DBS stent is 8 Fr guiding-catheter compatible 0.086” (Figure 1, B and C).

Figure 1. The DBS bifurcated coronary stent, a slotted stainless steel tubular balloon expandable stent with two asymmetric legs (A through C), deployed (A and C) or crimped on two balloons (B).
Procedural angioplasty
All patients were pretreated with daily aspirin (250 mg) and ticlopidine (500 mg) or a loading dose of clopidogrel (300 mg) the day before the procedure. During the procedure, heparin was administrated to achieve an activated clotting time ranging between 250 and 300 seconds. The procedures were performed via a femoral approach using an 8 Fr introducer. Prior to dilatation, two wires (0.014”) of 250 cm length were placed in the distal bed of each branch of the bifurcation. Each lesion was predilated with a semi-compliant balloon catheter. The size of the balloon catheter was chosen to reach a balloon/artery ratio close to 1, for each branch. Predilatation was completed by double balloon inflation (kissing technique). Then, the DBS delivery system was advanced over both guidewires towards the bifurcation and was correctly positioned on the carina. The stent was deployed at a pressure of 10 atm in each balloon, beginning by the SB balloon, and ending by inflation of the MB balloon. Finally, the kissing balloon technique was performed (Figure 2).
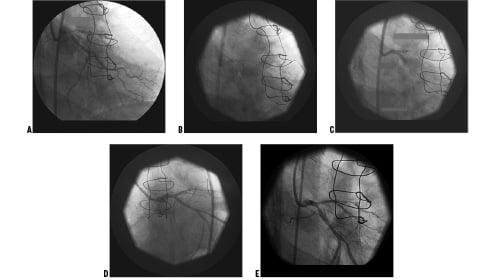
Figure 2. Protected distal tight left main bifurcated lesion angioplasty with a DBS stent (A through E). Before angioplasty (A), stent positioning at the carina after predilatation with kissing balloon procedure (B), DBS stent deployment (C), final result (D) and at 6 months angiographic follow-up without significant restenosis (E).
GP IIb/IIIa inhibitors were let at the investigator’s preference. Patients received their usual treatment with a daily dose of aspirin (250 mg) plus ticlopidine (500 mg) or clopidogrel (75 mg) for 30 days.
Follow-up
Clinical evaluation follow-up with ECG was obtained at 30 days, six months and five years in all patients. Coronary angiographic assessment was required at six months in all patients with angiographic procedural success (Figure 2). Coronary angiography could be performed earlier when clinically indicated.
Quantitative angiographic analysis
Cine-angiogram parameters were obtained for the target lesion before the procedure, immediately after the procedure and at six months follow-up according to standard acquisition guidelines. Angiograms were submitted to an independent core angiography laboratory (Corisis, Saint Denis, France), and were analysed using a computer-assisted, automated edge detection algorithm (CMS 4.0, MEDIS, Leiden, The Netherlands). RD and minimal lumen diameter (MLD) were used to calculate the percentage of stenosis. The acute gain, net gain and late luminal loss were defined as follow:
Acute gain (mm)=post PTCA MLD – initial MLD.
Net gain (mm)=final MLD – initial MLD.
Late luminal loss (mm)=post PTCA MLD – final MLD.
For the purpose of the present analysis, bifurcated lesions were divided in three segments (MB proximal: segment I, distal: segment II, SB: segment III) and each of those was considered as an independent lesion (Figure 3).
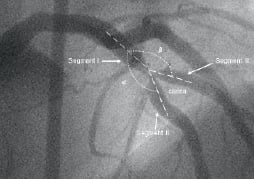
Figure 3. Angiographic view of a bifurcated lesion. Angle of the carina equal 360° - (α MB + β SB); MB, main branch; SB, side branch.
RD of each segment was used for calculation of percent diameter stenosis. In order to analyse the DBS conformation to the coronary anatomy, the angles formed by these three segments (Figure 3) were measured before and after stent implantation, using commercially available software (OSIRIS PPC 3.5, University of Geneva, Switzerland).
Endpoints and definitions
The primary endpoint was procedural success, defined as adequate DBS stent delivery and deployment evaluated on a residual diameter stenosis inferior to 30% as measured by quantitative coronary angiography (QCA), a TIMI 3 flow on the two branches and absence of MACE at day 30. MACE was defined as the occurrence of death, Q-wave or non Q-wave myocardial infarction and/or myocardial target vessel revascularisation (TVR) with repeat PTCA or coronary artery bypass grafting (CABG).
The secondary endpoints included the six months angiographic binary restenosis (defined by QCA as a residual stenosis > 50%), MACE at six months and five years and recurrent angina pectoris.
Statistical analysis
Continuous variables are expressed as mean±SD. Univariate comparisons were made using the chi-square test with Yates correction for categorical variables, an independent student’s t-test for comparison of two continuous variables and an ANOVA for comparison of the three segments. A binary logistic regression analysis evaluated factors associated with restenosis at six-months follow-up. Kaplan-Meier survival distribution function estimate was calculated for target lesion revascularisation (TLR) and MACE (SPSS 12.01, Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Results
Patients and lesions characteristics
Between April 2000 and July 2001, 34 patients were enrolled in the present study. Patient characteristics of this population are listed in Table 1.
The majority of culprit lesions were located on LAD / diagonal bifurcation followed by distal protected LM, LCX/obtuse marginal and distal RCA/posterior descending coronary artery. Lesions were often eccentric and little calcified. Lesion characteristics are presented in Table 2.
According to the Duke University classification the majority of lesions were true bifurcations defined as D lesions (Table 2), stenosis including the parent vessel and ostium of the SB28. Angiographic lesion characteristics are shown in Table 2.
Procedure outcome and clinical results at one month
Mean duration of the procedure was 76±25 min (range values 35 to 125 min). Thirty-two patients out of 34 (94%) were successfully implanted with a DBS. Among these 32 patients, MB and SB TIMI 3 flow was obtained in all cases. Uncomplicated failures were observed in two cases. In the first case, the DBS stent could not pass an eccentric and heavily calcified lesion despite a successful balloon pre-dilatation on the LAD. In the second case, the main and side legs of the DBS stent were wrongly positioned on the two guidewires and thus inverted. The DBS could not reach the lesions and the device was safely pulled back. In both cases, a commercially available tubular stent was implanted onto the MB and completed by a successful kissing balloon technique.
Additional stenting was necessary in 10 cases in order to cover the distal part of the bifurcation lesion (n=4) or to dilate another coronary lesion (n=6). Procedural characteristics of the patients are summarised in Table 3.
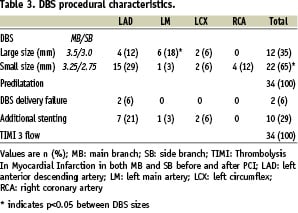
No myocardial infarction occurred. At one-month, no other MACE or acute or sub-acute stent thrombosis was reported. Thus, the primary endpoint has been reached in 32 cases (94%).
QCA results
All patients had a successful procedure defined as a final stenosis of less than 30% in the proximal segment I. A small residual stenosis on segment II was observed in three patients (43%, 33% and 36% respectively), and on segment III in two other patients (40% and 35% respectively). Table 4 shows the quantitative analysis of each segment before the procedure, immediately after the procedure and at the six months follow-up.

Segment I had larger RD at baseline immediately after the procedure as compared to other branches (p<0.01). Post-procedural MLD, as well as acute gain, were also more notable in segment I (p<0.01). Balloon/artery ratio was lower in segment I than in other segments (p<0.05).
Follow-up angiography was achieved in 29 patients (91%) at a mean of 6±1 months following the initial procedure.
The best results regarding MLD, percent stenosis and net gain were obtained in segment I at six months (p< 0.01). Late luminal loss was more important in segments II than in segment I (p<0.01). Angiographic binary restenosis was more frequently observed in segment II and III than in segment I (p<0.05). Five patients had restenosis involving both segments II and III. According to the effective treatment, the overall binary restenosis rate observed at any part of the DBS stent was 34% (10 out of 29 patients with angiographic data available at six months).
Figure 4 shows the cumulative frequency of stenosis immediately after the procedure and at six months in each segment.
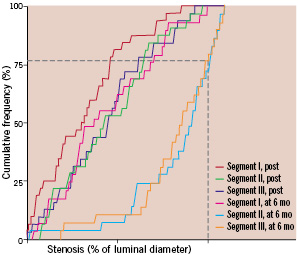
Figure 4. Cumulative frequency of stenosis immediately after stenting (post) and at 6 -months (mo) follow-up in patients who received DBS stent (segment I is the main segment, segment II is the secondary segment located on the main branch, segment III is the side branch). The broken lines indicate the percentage of side branch restenosis (above the line, 28% segment II and 24% segment III).
There was a marked difference between segment I and the other segments at six-months follow-up. Among the seven patients with segment III restenosis, five had a significant stenosis before the initial procedure. Non-significant diseased SB (< 50% diameter stenosis) were stented in 15 cases. Among these patients, a significant stenosis was observed at 6-month’s follow-up in two cases.
Among the seven patients with a stenosis of a distal protected LM artery, only one experienced a restenosis involving segment II and III but not segment I.
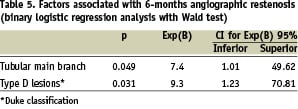
Mean values of angles did not differ after stenting. A very close relation between the values of the angle of the carina before and after DBS deployment was observed (67±18 compared with 63±19 degrees, respectively, R coefficient=0.86, p < 0.01 for the linear regression analysis). Maximum carina angle value was 108 degrees before and 88 degrees after stent deployment. On binary regression analysis, only tubular lesion of the MB as well as type D lesion according to the Duke classification were variables associated with restenosis at six-months follow-up (Table 5).
Late clinical adverse events
Cumulative in-hospital MACE, at six months and at five years are displayed in Table 6.
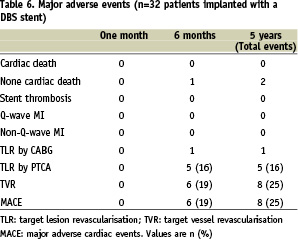
One patient experienced recurrent angina at five months due to angiographic restenosis in the two distal parts of the DBS stent and a de novo lesion. CABG was considered for this patient but he died prematurely after coronary angiography from shock due to a massive groin haemorrhage. The other five patients requiring TLR at six months were treated by repeat PTCA. Two patients died from cancer two years and five years after DBS implantation, respectively. One patient died from cerebrovascular accident two years after implantation. One patient had non-target vessel revascularisation (circumflex artery) three years after DBS implantation on a LAD/diagonal bifurcation. TVR concerned two other patients, at 18 and 24 months, with de novo lesions located at a distance > 1 cm from the distal part of the DBS stent, but without restenosis on the DBS. Kaplan-Meier estimates of event-free survival curves are shown in Figure 5.
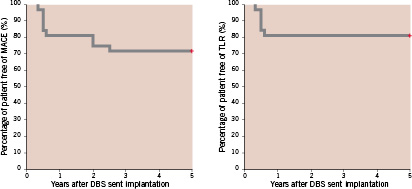
Figure 5. Kaplan-Meier curves showing survival time free of major adverse cardiac events (MACE) and target lesion revascularisation (TLR) at 5 years follow-up in patients who received a DBS Stent.
Intention-to-treat analysis showed that TLR was needed in six (19%) patients. The rate of freedom from MACE which consisted only in TVR at five years was 75% (24/32 patients).
Discussion
The major finding of this study was the demonstration of feasibility and safety of the treatment of true bifurcation lesions involving two large coronary branches using a true bifurcated stent. The percentage of successful implantation (94%) was high with this first generation device. Neither stent thrombosis, nor acute MI was observed. At six months the binary overall restenosis rate was 34%, without restenosis in the proximal part of the stent and the late luminal loss is comparable to non-bifurcation lesions treated with other bare metal stents. We observed a promising result in the subgroup of seven patients treated for distal protected LM stenosis in which only one case experienced restenosis in the distal part of the stent.
Moreover, seventy-five percent of patients were free of symptom and cardiac events at five years follow-up, and none experienced late stent thrombosis. This data confirmed the long-term safety of the device.
Measurement of angles before and after the procedure showed that the bifurcation morphology was preserved, due to the conformability of the two distal branches of the DBS stent. The large diameter of the MB which induces a high acute gain and the adequate stent placement across the bifurcation, may explain the relatively low rate of restenosis observed in these complex lesions. The efficient scaffolding of the SB ostium without overlapping or gap between the two parts of the stent warrants ostium patency. This concept obviates the risk of the “jail effect” toward the SB ostium and allows large SB access and easy re-crossing. Both patients who presented with downstream de novo lesions were easily treated by balloon catheter dilatation and/or stenting through the initial DBS stent.
Prior to this first human clinical trial, three years of in vitro testing and experiments on porcine model, with both clinical and one to seven month angiographic follow-up, were needed to finalise the design of the stent and to demonstrate the feasibility, efficacy and safety of this device19. The concept of a stent dedicated to the bifurcation appeared in the literature a few years ago, but no systematic angiographic evaluation of the restenosis rate at six months were performed and low rate of successful device implantation were reported due to their inadequate shape20-22. More interesting were the approach with stents incorporating a side aperture or a very limited amount of scaffolding for the side-branch-ostium23-25. A high rate (43%) of side-branch additional stenting and a 45% overall restenosis rate for any branch was reported in the Frontier Stent Registry24. At six months the MACE rate was 17%, similar to our results.
Even in the DES era, the SB remained a problem (21% of restenosis as compared to 6% of restenosis in the MB) and no indications were given regarding the most appropriate technique, i.e. stenting of both branches or stenting of the MB with provisional stenting of the SB29. Tanabe et al reported a 10% incidence of MACE at six months with sirolimus-eluting stent implantation in both the main vessel and SB30. Two randomised studies showed that elective SB stenting seems to provide no advantages over the simple stent jail followed by SB balloon dilation31,32.
The major complication of coronary stenting remains acute thrombosis which is associated with either death or nonfatal myocardial infarction33,34. Farb et al have described, among the mechanisms of late stent thrombosis, the improper position of the SB stent which protruded back into the MB. This complication seems to be more frequent in bifurcated lesions than in other type of complex lesions. This is particularly true with the use of DES in treatment of coronary bifurcation lesions. Colombo et al reported a 3.5% risk of stent thrombosis in their randomised study29. In the present study, we did not observe any DBS stent thrombosis.
Study limitations
The major limitations of this study were the use of bare metal stents in the era of DES and the relatively small number of patients included in this pilot-trial. The bulk and tractability of the DBS stent need to be improved to allow a smaller size catheter approach. However, the present study is a step toward a safe and efficacious treatment of bifurcated lesions involving two large coronary vessels.
Conclusions
The use of a true bifurcated stent with two legs in the treatment of bifurcated lesions of large coronary arteries appears feasible and safe with a high successful implantation rate, no late thrombosis, a relatively low restenosis rate at six months, stable clinical results and low MACE at five years. This bifurcated device should be improved and appears as an efficient platform for a future DES.
Appendix
The following institutions and investigators participated in the FIH DBS study. The number of patients enrolled is given in brackets.
Institut Mutualiste Montsouris, Paris (12): A. Dibie; Centre Cardiologique du Nord, Saint-Denis (11): B. Chevalier; Clinique Pasteur, Toulouse (7): J. Fajadet; Institut Jacques Cartier, Massy (4): T. Lefèvre.
Acknowledgements
The authors gratefully acknowledge the help of Dr. Jean Bachet for editorial assistance.
This study was supported by a grant from Cordis, a Johnson & Johnson company.

