Abstract
Aims: We report 2-year outcomes in a large unselected drug-eluting stent population (N=7,492) in the TAXUS Express2 ARRIVE post-market surveillance programme (101 U.S. sites).
Methods and results: No specific inclusion/exclusion criteria were mandated; patients enrolled at procedure initiation. Two-year follow-up was 94%, with independent adjudication of major cardiac events, monitoring of patients with cardiac events and an additional 10-20% sample by site. Most ARRIVE cases (64%, n=4,794) typified expandeduse based on patient/lesion characteristics outside the simpleuse (single vessel/stent) pivotal trial populations. These expanded use patients had higher 2-year rates than simpleuse patients for mortality (7.8% vs. 4.2%, P<0.001), myocardial infarction (MI, 3.9% vs. 2.2%, P<0.001), target lesion revascularisation (TLR, 9.2% vs. 5.4%, P<0.001), and stent thrombosis (3.3% vs. 1.4%, P<0.001). Among subgroups with renal disease, chronic total occlusion (CTO), lesion >28mm, reference vessel diameter (RVD) <2.5 mm, multivessel stenting, acute MI, bifurcation, vein graft, or in-stent restenosis, TLR ranged from 3.8% to 8.9% in yearone, and from 1.3% to 6.0% during yeartwo.
Conclusions: Mortality and stent-related events were higher in expanded use than simple use patients in the pivotal trials. ARRIVE provides a detailed estimate of procedural and 2-year outcomes in such real-world patients.
Introduction
Randomised, controlled trials (RCT) have demonstrated a clear restenosis advantage of drug-eluting stents (DES) over bare-metal stents (BMS), with no significant difference in death or myocardial infarction (MI) in the generally uncomplicated patients/lesions studied in such trials.1-4 However, the inherent homogeneity of RCT patients limits the ability to extrapolate their findings to routine practice that involves a broader range of clinical conditions and lesion types. The U.S. Food and Drug Administration (FDA) thus mandated as a condition for approval of the paclitaxel-eluting TAXUS™ Express2™ stent (PES) the systematic collection of data through 2-years among unselected patients. The TAXUS peri-AppRoval Registry: a multIcentre safety surVEillance (ARRIVE) programme was undertaken as a two-phase U.S. safety surveillance registry at 103sites. With 2-year data on 7,492 patients, including 4,794 patients who would have been excluded from pivotal RCTs, ARRIVE allows detailed estimation of even low frequency stent related events in a real-world DES population.
Methods
Patient selection, device, study procedure
The two registries –FDA-mandated ARRIVE 1 and the voluntary post-market ARRIVE 2– were similarly designed to consecutively enrol patients, as described previously.5 Both are registered on the National Institutes of Health website (Identifiers NCT00569491 and NCT00569751). Consecutive enrolment was defined as a commitment by ≥2 investigators at each facility to enrol all consented patients deemed appropriate for a DES. Patients provided informed consent under a protocol approved by the local institutional review board in conformity with the Declaration of Helsinki and FDA guidelines. No specific inclusion/exclusion criteria were mandated; all patients receiving a TAXUS stent were included, whether or not they also received a non-TAXUS stent during the index procedure. Each patient was enrolled at procedure initiation to minimise potential bias by exclusion for complicated or unsuccessful procedures. The TAXUS Express2 PES (Boston Scientific Corporation [BSC], Natick, Massachusetts, USA) has been described previously.6 Vessel size and lesion length were determined by visual estimate; stents were placed per the Directions For Use (DFU) and/or standard percutaneous coronary intervention (PCI) practices. Dual antiplatelet therapy (aspirin and clopidogrel/ticlopidine, DAPT) was begun before or immediately after the procedure. Aspirin was continued indefinitely with oral clopidogrel/ticlopidine recommended for sixmonths per the DFU.
Data collection, monitoring, follow-up
Data, captured via webbased reporting with predefined queries, were verified against source documents for all cardiac events. To encourage accuracy and completeness of data collection, sponsor (BSC) monitors assessed an additional 20% per site sampling of patients in ARRIVE 1 and 10% in ARRIVE 2. A Clinical Events Committee (CEC, Appendix 1 - online as supplementary data at www.eurointervention.org) independent of BSC determined the relationship of reported cardiac events to the study device. An event was considered “TAXUS-stent-related” if it occurred at the stented segment or if the relationship to the TAXUS stent could not be excluded based upon available information.
Study definitions are in Appendix2 (online as supplementary data at www.eurointervention.org). Major cardiac events (MCE) included all cardiac death, MI, and target vessel revascularisation (TVR). Follow-up angiography was not mandated, and was performed in accordance with local practice. Target lesion revascularisation (TLR) was defined as “TAXUS-stent-related” TVR, given the absence of a central angiographic core laboratory. An independent committee at the Harvard Clinical Research Institute adjudicated stent thrombosis (ST) per the ARC definite/probable definitions.7
Statistical analysis
Statistical analyses were carried out using the CEC’s assessment of “TAXUS-related” cardiac events. Analyses were assessed in a collaborative effort between the study principal and co-principal investigators and the sponsor. Patient, lesion, and procedural characteristics and event rates were analysed using descriptive statistics with SAS System Software, Version 8.0 or higher (SAS Institute, Cary, North Carolina, USA). Simple proportions with two-sided Pvalues from Student t-test were used for continuous variables; chi-square test was used for noncontinuous variables. The Kaplan-Meier product method (log-rank Pvalue) was used for time-to-event analyses. To identify predictors of major events at 2years, 41 variables (Appendix 3 online as supplementary data at www.eurointervention.org) were assessed using backward Cox proportional hazards regression; the threshold to remain in the model was P=0.10.
Results
Patient, lesion, and procedural characteristics
Patients were enrolled February through May2004 (ARRIVE 1) and October2004 to October2005 (ARRIVE 2). The two ARRIVE registries (Appendix 4 online as supplementary data at www.eurointervention.org) were designed to allow data pooling and tested for its appropriateness, and together comprised an analysis population of 7,492 patients (Table 1).

Most ARRIVE cases (64%) were classified as expanded use (n=4,794, Figure 1) based on patient and/or lesion characteristics considered outside the simple use population studied in the TAXUS IV pivotal RCT.3
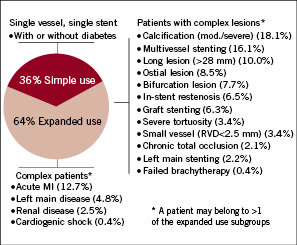
Figure 1. Usage patterns in ARRIVE (N=7492). Simple use cases excluded one or more of the following: acute myocardial infarction (AMI); bifurcation, cardiogenic shock, chronic total occlusion, prior brachytherapy, vein graft stenting, in-stent restenosis, large vessel (RVD>3.75 mm), left main disease/stenting, long lesion (>28 mm), moderate/severe calcification, multivessel stenting (mean of 2.1 vessels per patient), ostial lesion, renal disease (serum creatinine >3.0mg/dL or dialysis), severe tortuosity, small vessel (RVD<2.5 mm). Expanded use cases are those not classified as simple use.
Baseline characteristics and a comparison between simple and expanded use cohorts are shown in Table 2.

Expanded use had statistically significantly more baseline comorbidities as well as more complex disease than simple use (Table 2). Characteristics of nine expanded use subgroups show the higher baseline risk associated with these patients (Table 3).
Outcomes in year1 and year2
Outcomes data were available for 97% and 94% of analysed patients at 1-year and 2-years, respectively.In yearone, outcomes data were available for 7,274 patients and the 1-year per patient composite MCE rate was 9.5% (691/7274). This included cardiac death (2.2%, 159/7274), MI (2.1%, 155/7274), TVR (6.8%, 492/7274), and TLR (5.1%, 373/7274). In yeartwo, outcomes data were available for 6,882 patients and MCE occurred in 4.7% (325/6882), consisting mostly of TVR (3.2%, 223/6882) with a TLR rate of 2.5% (172/6882). All-cause mortality was 3.5% (257/7274) in yearone and 3.0% (204/6882) in yeartwo, with low rates of year two cardiac death (1.5%, 101/6882) and MI (1.1%, 74/6882). Stent thrombosis was 1.8% (128/7274) during year one and 0.8% (56/6882) in year two; at physician discretion 67.7% of patients received DAPT through 1-year, 53.1% through 2-years.
Diabetes status was determined by the investigative sites and 2,368 (31.6%) of all ARRIVE patients were considered diabetic. Medically treated (oral medications and/or insulin) diabetic patients made up 89.2% (2112/2368) of the ARRIVE diabetic population and outcomes data were available for 2,049 medically treated patients in yearone and 1,900 in yeartwo. These medically treated diabetic patients had a 1 year composite MCE rate of 10.7% (220/2049) with a TLR rate of 4.8% (98/2049). In yeartwo, their composite MCE rate was 6.6% (125/1900), with 3.1% TLR (59/1900). A detailed discussion of ARRIVE diabetic patients is the subject of a separate manuscript.8
Outcomes in expanded use subgroups
The ARRIVE expanded use cohort had significantly higher 2-year MCE rates compared to the simple use group (Figure 2).
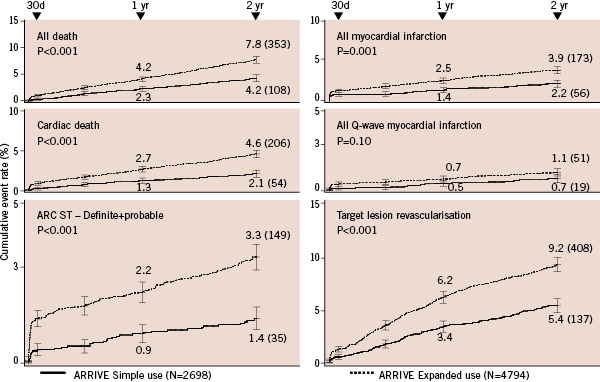
Figure 2. Comparison of event rates through 2-years in simple use and expanded use subgroups in ARRIVE. Definitions of simple use and expanded use are provided in Figure1. ARC ST definite/probable definitions are from Cutlip et al7. Target lesion revascularisation was defined as “TAXUS-stent-related” target vessel revascularisation, given the absence of a central angiographic core laboratory. Pvalue is log-rank; error bars are ±1.5SE. ARC: Academic Research Consortium; ST: stent thrombosis.
The most common event was revascularisation. Rates for early ST (≤30days) were >3-fold higher for expanded use (1.4% vs. 0.4%). Differences between incidence curves are evident before 30 days and continue through 2-years.
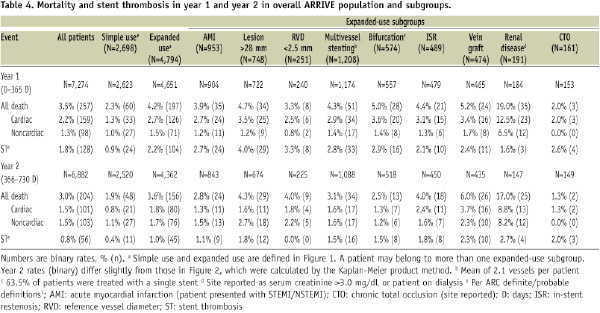
Mortality and ST were usually higher in yearone than yeartwo for the simple use and nineexpanded use subgroups; only subgroups with vein grafts and small vessels had higher death rates in yeartwo (Table 4).
Renal disease patients (serum creatinine >3.0mg/dL or dialysis) had more very late ST (VLST) in the second year and the highest combined rate for all death and MI (35.3%) through 2-years, significantly higher than the 6.0% rate in simple use patients (P <0.001, Figure 3).

Figure3. Comparison of Kaplan-Meier curves for combined all death and myocardial infarction between the ARRIVE simple use subgroup and the renal disease subgroup. Simple use and renal disease are defined in Figure1. P value is log-rank; error bars are ±1.5SE.
Rates for Q-wave MI in yearone were highest among patients with long lesions (>28 mm), small vessels (RVD <2.5 mm), and multivessel stenting (mean of 2.1vessels per patient) while the acute myocardial infarction (AMI) subgroup was below the 0.5% simple use rate (Figure 4).
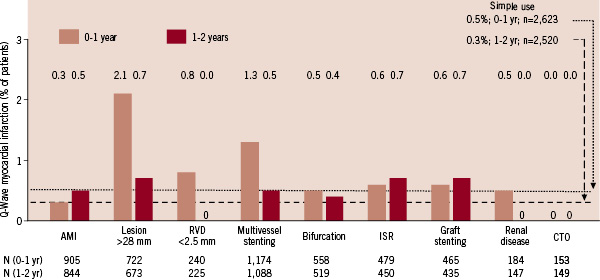
Figure4. Q-wave myocardial infarction in ARRIVE expanded use subgroups at 1-year and 2-years. Event rates presented here were calculated as simple proportions. Rates for simple use (defined in Figure1) differ slightly from those in Figure2, which were calculated by the Kaplan-Meier product method. AMI: acute myocardial infarction; CTO: chronic total occlusion; ISR: in-stent restenosis; RVD: reference vessel diameter.
During yeartwo most expanded use subgroups had higher Q-wave MI rates than simple use (0.3%) except small vessel, renal disease, and CTO, which had no events.
Figure 5 shows all expanded use subgroups had higher first year rates for TLR than simple use (3.4%). In yeartwo rates were lower than yearone for all subgroups except CTO. The AMI and small vessel subgroups had yeartwo rates below that of simple use (1.9%).
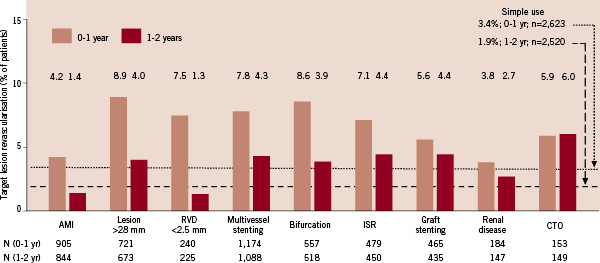
Figure5. Target lesion revascularisation in ARRIVE expanded use subgroups at 1-year and 2-years. Target lesion revascularisation (TLR) was defined as “TAXUS-stent-related” target vessel revascularisation, given the absence of a central angiographic core laboratory. Event rates presented here were calculated as simple proportions. Rates for simple use (defined in Figure1) differ slightly from those in Figure2, which were calculated by the Kaplan-Meier product method. AMI: acute myocardial infarction; CTO: chronic total occlusion; ISR: in-stent restenosis; RVD: reference vessel diameter
Multivariate predictors of adverse events
Table 5 shows multivariate predictors for accumulated events overthe twoyears of the registry, including all death, cardiac death, MI, and TLR.
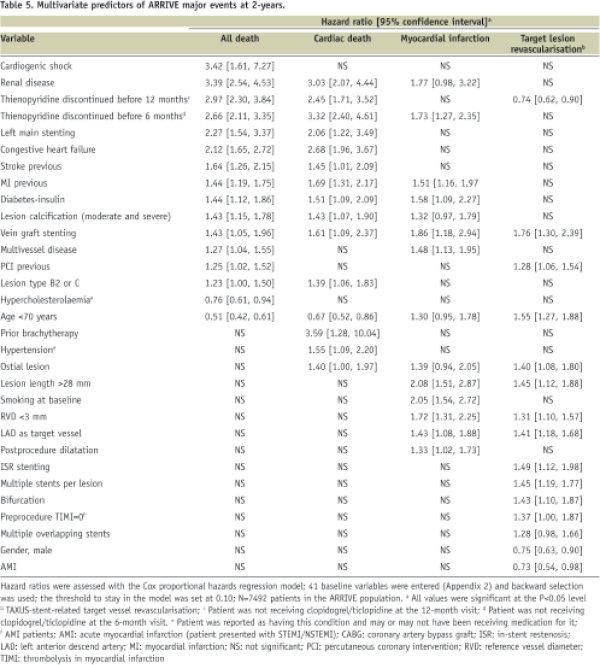
Renal disease was a strong predictor of death (3.4-fold increased risk), cardiac death (3.0-fold), and MI (1.8-fold). Other strong baseline predictors of death included cardiogenic shock (3.4-fold) and discontinuation of thienopyridine therapy before six (2.7-fold) or 12months (3.0-fold). Expanded use patients generally had an increased risk of death, cardiac death, or MI (1.3 to 3.6-fold per factor) and complex lesion characteristics increased TLR risk (1.3 to 1.8-fold per factor). Multivariate ST predictors included early discontinuation of thienopyridine therapy, baseline smoking, vessel RVD <3.0mm, prior brachytherapy, renal disease (>3-fold increased risk each) and others. A detailed discussion of ST and its predictors in ARRIVE is the subject of a separate manuscript.9
Discussion
The ARRIVE programme captured usage patterns and 2-year outcomes of the TAXUS Express2 stent in 7,492 patients treated during routine practice. Event rates were generally higher in year onethan yeartwo. Simple use cumulative mortality rates through one and two years mirrored those of similar patients enrolled in the RCT TAXUS arm (2.0%; 3.4%)5, validating the high degree of event ascertainment in the ARRIVE programme. Notwithstanding some overlap among patient subsets, analysis of ninespecific ARRIVE expanded use subgroups provide insight into treatment and outcomes of such high-risk patients.
Outcomes in routine practice-overall population
ARRIVE event rates remained consistent with those of a revascularised broad coronary disease population through 2-years. Mortality (3.5% in yearone and 3.0% in yeartwo) was similar to that reported for the National Heart, Lung, and Blood Institute Dynamic Registry (NHLBI),10 and the REAL, Ontario, and STENT registries.11-13 While ARRIVE ST rates(1.8% in yearone; 0.8% in yeartwo) were higher than that reported for STENT through 2-years (1.5% for off-label DES use), they were similar to the 18month ST rate of 2.9% for PES reported in the SORTOUTII randomised trial14 that enrolled patients from everyday clinical practice. The initial concentration of ST in the first 30days has been observed in other DES registries13,15,16 and with BMStreatment17 of complex lesions.
Data from ARRIVE and other large, multicentre registries provide practical estimates of event rates in DES patients for expanded use indications. This is highly relevant to clinical practice since “off-label” DES use (ST elevation MI, in-stent restenosis [ISR], bypass grafts, and CTO) accounted for 21.4% of the >400,000 DES procedures in the American College of Cardiology National Cardiovascular Data Registry for 2003-2004.18 Differences in the definition of “off-label” notwithstanding, ARRIVE’s 64% expanded use rate is similar to DEScover (47%),19 EVENT (55%),15 NHLBI (49%),20 and STENT (59%).13 As confirmed in ARRIVE, expanded use DES patients had significantly higher 1-year rates versus simple use for death, TVR, and ST in DEScover,19 more TLR and ST in EVENT,15 higher 1-year rates for death, MI, and revascularisation in NHLBI,20 and higher event rates through 2-years in STENT.13 Such differential outcomes have also been reported for sirolimus-eluting stent-treated populations.21
Comparisons of outcomes with DES and BMS have evaluated the use of DES in off-label use collectively and in select subgroups.11-13,20,22,23 Through 2-years, off-label DES use had a significantly lower TVR risk than BMS with a similar safety profile in NHLBI20 and among 3,751 pairs of propensity score matched patients in the Ontario registry.12 Mortality, MI, and TVR were also lower at ninemonths with off-label use of DES versus BMS in STENT, though the significant TVR advantage diminished by 2-years.13 Mortality through 4-years was significantly lower with DES compared to BMS in a single-centre study of 8,032patients undergoing PCI in 2003–2007.22
Outcomes in high risk subgroups
The large size of ARRIVE allowed for adequate numbers of specific highrisk subgroups. Though many overlap, their analysis can provide insight into current use as well as outcomes and help physicians estimate likely clinical outcomes for individual patients. In evaluating expanded use patients, one notes that many would be poor candidates for BMS due to high restenosis rates and more likely to be treated by coronary artery bypass grafting (CABG). In the ARRIVE multivessel stenting subgroup, repeat revascularisation (7.8% in yearone; 4.3% in yeartwo) was higher than that reported for patients undergoing CABG in the New York State (U.S.) registry (5.1% at 18months) but 1-year mortality (4.3%) was comparable to similar registry patients (4.2% for 2-vessel disease)24 and to patients in the CABG arm of the SYNTAX RCT (3.4%).25
ARRIVE outcomes tended to mirror earlier reports on select subsets. Among AMI patients, mortality (3.9% in yearone; 2.8% in yeartwo) and revascularisation (4.2% in yearone; 1.4% in yeartwo) were lower than that reported for similar patients in STENT (8.0% death and 8.0% TVR through 2-years),26 and a multicentre Massachusetts (USA) registry (10.7% death and 9.6% TVR through 2-years),27 but comparable to a single centre report.28 In the ARRIVE bifurcation subgroup, 2-year mortality was comparable to STENT13; yearone cardiac death was higher (3.6% vs. 2.0%) and TLR rates lower (8.6% vs. 15.3%) than PES rates in a small multicentre European registry.29 Mortality in ARRIVE compared to STENT was somewhat higher among patients with long (>28 mm) lesions and similar for patients receiving stents for ISR or CTO.13 Treatment of CTOs with PES (N=48) has been shown to significantly reduce 1-year MACE and restenosis rates compared to matched cases treated with BMS.30 Unlike the other eight ARRIVE subgroups, however, CTO subgroup TLR rates did not drop over time, as has also been reported for SES.31 Low revascularisation and high mortality in ARRIVE renal disease patients echoed other reports, including NHLBI where 1-year mortality was lower in those patients treated with DES than BMS.32,33
Mortality increased from yearone to yeartwo among ARRIVE patients receiving stents for vein grafts (5.2% vs. 6.0%) or small (<2.5mm) vessels (3.3% vs. 4.0%). Among veingraft patients, 2-year mortality was also high in STENT (8.8% at 2-years).13 With the sirolimus-eluting stent little difference has been reported between DES and BMS in long-term outcomes in veingraft stenting, despite possible increased late events.34-36 However, in the recently reported stenting of saphenous vein grafts RCT (BMS vs. PES, N=80), during a median follow-up period of 1.5 years PES were associated with significantly lower rates of angiographic restenosis, TLR, and target vessel failure.37 While small vessel size generally has not increased the risk of death or MI, it has been reported to increase revascularisation.38 In the ARRIVE small vessel group, 1-year TLR rates (7.5%) were lower than reported 9month rates among PES patients with RVD<2.41mm (16.4%) or RVD>2.41 and <2.84 mm (9.7%) in a single-centre DES registry,38 and small vessel TLR in year2 (1.3%) was the lowest of all ARRIVE subgroups, including simple use (1.9%).
Multivariate predictors of clinical events
Several characteristics of ARRIVE, including recruitment from multiple centres, specific predefined event definitions, use of a CEC, adequate independent monitoring, 2-year follow-up, and inclusion of all DES patient subsets allowed development of event predictors with substantial power. Renal disease was a strong (3.4-fold increased risk) independent predictor of mortality along with several other comorbid clinical factors, as reported by others.23,28,39 Early discontinuation of thienopyridine therapy was associated with increased mortality through twoyears in ARRIVE as also shown by Eisenstein et al who found mortality to be lowest among patients remaining on clopidogrel for at least oneyear.40 Iakovou et al41 identified early discontinuation of antiplatelet therapy as a predictor of early and late ST, as seen also in ARRIVE. Predictors of revascularisation in ARRIVE included lesionrelated factors similar to other studies.11,42 Awareness of the range of rates and significant predictors of MCE in DES registries may help physicians estimate individual patient outcomes and tailor treatment regarding choice of stent and follow-up regimen.
Study limitations
ARRIVE has some of the limitations common to registries, including absence of a control group, use of site visual assessments of angiographic data, absence of serial cardiac enzyme or electrocardiographic measurements that could underestimate the rate of smaller (non-Q) MI, and less monitoring than standard for traditional RCTs. Absent angiographic core laboratory evaluation, we cannot be certain that site reported «TLR» in the more extensive lesion subgroups fully excluded revascularisation events driven by progressive disease outside of the stented segment.43 However, the close concordance between ARRIVE and RCT rates supports the premise that a real-world registry with this level of monitoring can provide reliable ascertainment of critical adverse events during two years of follow-up after DES treatment and may be the only source of such information for the many complex patient subgroups that have not undergone RCT evaluation.
Conclusions
The ARRIVE registries capture the broad spectrum of disease routinely treated by percutaneous coronary intervention including 4,794(64%) expanded use cases outside the patient/lesion characteristics studied in pivotal RCTs. Rates for mortality and stentrelated events are expectedly higher in the expanded use cohort, and provide a valuable estimate of procedural and 2-year outcomes in such patients pending completion of ongoing RCTs.
Acknowledgments
The authors thank Yun Lu, M.S.; Kevin Najarian, M.S.; and Aijun Song, M.S. (BSC) for assistance with statistical analyses.

