Abstract
Aims: First generation DES have markedly reduced restenosis. However, there is a major interest in developing new DES with greater flexibility, radiopacity and safety profile. The Elixir Medical drug eluting stent is a novel DES that combines a chromium-cobalt platform with novolimus (an antiproliferative sirolimus-analogue drug) and a polymer from the methacrylate family. As potential advantages, it provides a lower drug dose as compared to Cypher® (85 µg of novolimus vs. 140 µg of sirolimus) and therefore has a lower polymer load. We sought to evaluate the safety and efficacy of this novel device in reducing neointimal hyperplasia as assessed by QCA and IVUS.
Methods and results: In April 2007 a consecutive cohort of patients with de novo lesions < 14 mm in length, located in native coronaries of diameter from 3.0 to 3.5 mm were consecutively enrolled in this First-In-Man study (FIM). By protocol, angiography and IVUS would be done at baseline and repeated at four and eight months. Dual anti-platelet therapy was maintained for a minimum of 12 months. The primary endpoint was QCA lumen loss at 4-month follow-up. Secondary endpoints included MACE, in-stent neointimal obstruction by IVUS and device success. A total of 15 patients were included with 67% female patients and diabetes was detected in 47% of the cohort. Angiographic and procedural success was achieved in all patients. At 4-month angiographic follow-up there was in-stent late lumen loss (0.15±0.29 mm) by QCA and % volume obstruction (2.6±2.6) by IVUS. The angiographic in-stent late lumen loss results at eight months were 0.31±0.25 mm and % volume obstruction by IVUS was 6.0±4.4%. Late incomplete stent apposition (ISA) were not observed among these patients and no MACE was evidenced through nine month clinical follow-up.
Conclusions: In this FIM study, implantation of the novolimus-eluting stent was proven to be feasible, safe and elicited minimum neointimal proliferation. Additional large clinical trials should be considered to confirm these promising results.
Introduction
Despite the undeniable efficacy of first-generation drug-eluting stents (DES) including Cypher™ (Cordis, Johnson & Johnson, Miami Lakes, FL, USA) and Taxus™ (Boston Scientific Corporation, Natick, MA, USA) in reducing neointimal proliferation and therefore the need for repeat revascularisation when compared to bare-metal stents (BMS)1-4, concerns regarding the long term safety have markedly impacted their widespread utilisation5-7.
As a consequence, there is a major interest in developing new DES systems with better safety profiles and also greater flexibility and radiopacity. Recently developed, the Elixir Medical Excella novolimus eluting coronary stent system (Excella Stent) combines a cobalt-chromium platform with a biocompatible methacrylate polymer and a low dose formulation of novolimus, a macrocyclic lactone with anti-proliferative properties.
We sought to investigate, for the first time in humans, the performance, safety, and efficacy of this next generation DES.
Methods
Study population
In April 2007, a consecutive cohort of patients with single, de novo lesions < 14 mm in length, located in native coronaries with diameters of 3.0 to 3.5 mm (by visual assessment) were enrolled in this First-In-Man (FIM) study. Patients presented with symptoms of angina or silent ischaemia clearly documented through non-invasive assessment. Patients treated within 72 hours of an acute myocardial infarction and/or requiring more than one stent to treat the target-lesion were excluded from this study. We also excluded patients with heavily calcified lesions, those with lesions at bifurcations or involving the ostium of the coronary vessel, patients with severe left ventricular dysfunction (LVF < 30%), those with visible thrombus and also with contraindication to any of the protocol medications.
The protocol was approved by the local Ethics committee and written inform consent was obtained from all patients before inclusion in the study.
Device description
The Elixir Medical Excella novolimus eluting coronary stent system is a next generation DES designed to optimise safety and efficacy through the combination of the stent platform, the polymer coating for controlled release and the pharmacological agent and is comprised of the delivery system and the novolimus-eluting stent with polymer coating.
Stent platform
The delivery system has a proximal stainless steel hypotube and distal nylon blend shaft with a nylon blend balloon. The premounted stent is made of cobalt chromium alloy and has a nominal strut thickness of 0.0032” (81 µm) and an 8 crown-2 link pattern designed to optimise vessel coverage and flexibility (Figure 1).
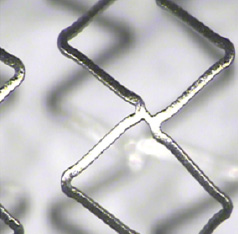
Figure 1. The chromium-cobalt platform used in the Excella-novolimus eluting stent. The eight-crown two-link design confers better scaffolding properties to this platform. The strut thickness of 0.0032” favours the flexibility and deliverability of the system.
Polymer coating
The purpose of the durable polymer coating is to moderate the delivery of the drug over a period of time. The polymer is from the methacrylate family of polymers, which is similar to the polymers used clinically on vascular implants including drug eluting stents such as Cypher™ (Cordis, Johnson & Johnson, Miami Lakes, FL, USA) and Resolute™ (Medtronic Vascular, Minneapolis, MN, USA) and which have been proven to be biocompatible over their long history of clinical use. The drug-polymer matrix is applied over the entire surface of the stent and allows sustained release of novolimus over a period of 4-6 weeks, and has a total thickness of ~3 µm which is substantially thinner than other commercially available DES devices using similar durable polymers.
Anti-proliferative agent: novolimus
Novolimus belongs to the family of compounds of macrocyclic lactones with immunosuppressive and anti-proliferative properties and has a similar mechanism of action to other macrocylic lactones such as rapamycin. Macrocyclic lactones bind to the immunophilin, FK Binding Protein-12 (FKBP-12), to generate an immunosuppressive complex. This complex binds to and inhibits the activation of the mammalian Target of Rapamycin (mTOR), a key regulatory kinase. This inhibition suppresses cytokine-driven cell proliferation, inhibiting the progression from the G1 to the S phase of the cell cycle. In vitro testing has demonstrated that IC50 of novolimus is approximately 0.5 nanomolar and compares well with rapamycin (0.1 nanomolar) in demonstrating high potency to inhibit hSMC proliferation. The dose of novolimus used in the Excella stent is approximately 5 mcg per mm of stent length.
Procedure
For the present study, the Excella novolimus eluting stent was only available in two diameters (3.0 and 3.5 mm) and a single length (18 mm).
All interventions were performed according to current standard guidelines. After mandatory pre-dilatation of the target lesion, stents were deployed, and as needed were post-dilated with high pressure guided by intravascular ultrasound (IVUS).
Dual antiplatelet therapy including a loading dose of aspirin (200 to 325 mg) and thienopyridine (clopidogrel 300 mg) was started 24 hours before the procedures. Post procedural aspirin was continued indefinitely and clopidogrel was maintained for twelve months (75 mg/day). During the procedure, intravenous heparin (70 to 100 IU per kg) was administered after sheath insertion to maintain an activated clotting time >250 seconds. Use of additional medications during the procedure, including glycoprotein IIb IIIa inhibitors, was left at the operator’s discretion.
A 12-lead electrocardiogram was obtained: (1) before the procedure, (2) immediately afterwards, and (3) 24 hours later. Blood sample laboratory analysis included creatine kinase (total CK and CK-MB subfraction) before procedure (<24 hours) and 12-18 hours after treatment.
Follow-up
After discharge, patients were clinically followed by medical appointment at one, four, six and eight months, with further clinical follow up at 12 and 24 months.
Two invasive follow-ups (angiography and IVUS) were scheduled for four and eight months, respectively.
Quantitative Coronary Angiography (QCA) analysis
Angiographic studies were performed at baseline, post-procedurally and at follow-up, in two orthogonal views after the intracoronary administration of 100-200 µg of nitroglycerin. The same angiographic angles performed at baseline were reproduced at the subsequent follow-up studies. Digital angiograms were analysed off line with the use of an automated edge-detection system (QCA-CMS, Medis Medical Imaging Systems, Leiden, The Netherlands).
Lesion morphology was assessed by using standard criteria, and lesion complexity defined according to the modified American College of Cardiology / American Heart Association (ACC/AHA) classification system. The contrast-filled catheter tip was used for calibration.
The quantitative angiographic parameters included: (1) reference vessel diameter; (2) Minimum Lumen Diameter (MLD); (3) lesion length; (4) percent diameter stenosis (difference between the reference diameter and MLD divided by the reference diameter and multiplied by 100); and (5) late luminal loss (difference between MLD at the end of the procedure and MLD at follow-up). Quantitative analysis was performed in the “in-stent” area (inside the stented segment) and in the “in-lesion” segment, including the stented area as well as 5 mm both proximal and distal to the stent. In-stent and in-lesion restenosis were defined as > 50% diameter stenosis at follow-up located within the stent and the target lesion respectively.
Intravascular Ultrasound (IVUS) analysis
IVUS studies were performed immediately post procedure and at the respective follow-ups, after an intracoronary administration of 100-200 µg of nitroglycerin.
All IVUS studies were performed with a motorised automatic transducer pullback system (0.5 mm/s) and commercially available scanners (i-Lab, Boston Scientific Corporation, Natick, MA, USA) consisting of a rotating 40-MHz transducer catheter (Atlantis SR pro, Boston Scientific Corporation, Natick, MA, USA) with a 2.6 Fr imaging sheath. The images were digitalised for off line quantitative analysis according to the American College of Cardiology’s Clinical Expert Consensus Document on IVUS. Quantitative IVUS analysis was made using a commercially available computerised planimetry program (EchoPlaque; INDEC Systems, Mountain View, CA, USA).
Quantitative parameters of the lumen, stent and vessel (external elastic membrane) cross-sectional areas were determined. Neointimal area was calculated as the stent area minus the lumen area at follow-up. Late lumen area loss was calculated as the minimum lumen area following initial stent deployment minus the minimum lumen area within the stented segment at follow-up. Lumen, stent, vessel and neointimal volumes were calculated using Simpson’s rule. Percent of neointimal volume obstruction was determined by the neointimal volume at follow-up divided by the follow-up stent volume and multiplied by 100. Neointimal hyperplasia (NIH) volume index was calculated dividing the total NIH volume by the stent length.
Stent incomplete apposition was defined as > 1 stent strut clearly separated from the vessel wall with evidence of blood speckles behind the struts, and was classified as: (1) persistent (when present both in the post-stent implantation and follow-up studies); (2) late acquired (when not present at post-stent implantation, but detectable at the follow-up study); and (3) resolved (when present at post-stent implantation, but not detectable at the follow-up study).
QCA and IVUS analyses were performed by an independent core laboratory, the Cardiovascular Core Analysis Laboratory at Stanford University, Stanford, CA, USA.
Study endpoints
The primary endpoint of this analysis was in-stent luminal late loss as measured by QCA, at four-month angiographic follow-up.
Secondary endpoints included acute success (angiographic and procedure success), cumulative rate of major adverse cardiac events (MACE) up to two years, rates of target-lesion (TLR) and target-vessel revascularisation (TVR) up to two years, and in-stent volumetric neointimal burden by IVUS at four and eight months.
Device success was defined by the presence of residual stenosis < 50% in the treated segment, in the presence of TIMI 3 flow following implantation of the Excella stent. Procedure success was defined by device success associated with no in-hospital major adverse events. MACE was defined as death, non-fatal myocardial infarction (Q and non Q wave) and need for repeat lesion revascularisation (by new percutaneous intervention or CABG). All deaths were considered cardiac unless a clear non-cardiac reason was identified. Myocardial infarction was defined by an increase in the creatine kinase-MB twice the upper normal limit with or without new Q waves on the ECG.
Statistical analysis
Continuous variables are expressed as mean±SD. Comparisons between post intervention and follow-up measurements were performed using a 2-tailed paired t test. A P < 0.05 was considered statistically significant.
Results
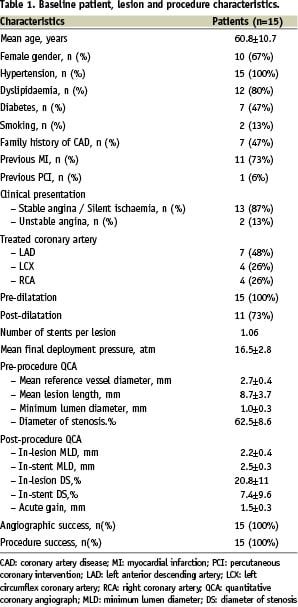
Out of the 15 consecutive patients included in this first-in-man analysis, 67% were women and the mean population age was 60.8 years. Diabetes mellitus was highly prevalent among these patients (47%). The LAD was the most frequently treated lesion (48%) and the mean reference vessel diameter and lesion length pre intervention were 2.7±0.4 mm and 8.7±3.7 mm, respectively. Table 1 contains a complete description of patient clinical and angiographic characteristics as well as procedure details.
All stents were successfully deployed and all patients were discharged without complications following the percutaneous intervention. Enzymatic elevations (CK and CK-MB) were not observed among these individuals.
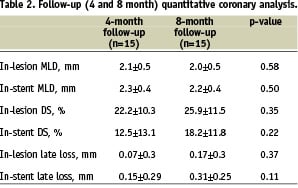
All fifteen patients underwent two invasive follow-ups. The study primary endpoint, 4-month in-stent late lumen loss, was 0.15±0.29 mm, while in-lesion analysis revealed a late loss of 0.07±0.3. Between four and eight months there was a non-significant increase of 0.16 mm in in-stent late lumen loss (p=0.11). Notably, there was no incidence of binary restenosis (diameter stenosis> 50%) among these patients. (Table 2)
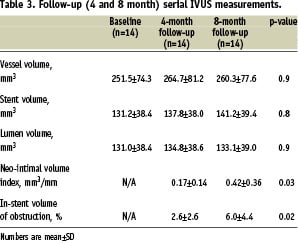
Serial IVUS analysis was available for only 14 patients. At four-months, the in-stent percent volume obstruction and neo-intimal volume index results were 2.6±2.6% and 0.17±0.14 mm3/mm, respectively. At eight months, final in-stent percent volume obstruction and neo-intimal volume index were 6.0±4.4% and 0.42±0.36 mm3/mm (p=0.02 and 0.03, respectively). Importantly, the vessel, stent and lumen volumes did not significantly vary from baseline to four and eight months. IVUS results are presented in Table 3.
A total of six cases of acute incomplete stent apposition were noted in the post intervention IVUS study. At eight month follow-up, one of these cases resolved and the remaining five were classified as persistent by the core lab. Late-acquired incomplete stent apposition was not evidenced in this cohort.
At the 8-month clinical follow-up, all patients were asymptomatic with negative non-invasive ischaemia tests. There were no major adverse clinical events, including cardiac death, non-fatal myocardial infarction and need for repeat lesion revascularisation. Additionally, all patients were free of cerebrovascular accident and stent thrombosis.
Discussion
The main finding of this first-in-man analysis of the novolimus-eluting stent is the possibility of using a lower dose of anti-proliferative drug, requiring less amount of polymer while still achieving excellent neointimal hyperplasia inhibition after percutaneous treatment of de novo coronary lesions.
Although not completely understood, pathological studies have correlated late and very late DES thrombosis to a local inflammatory reaction associated with exposed stent struts resulting from incomplete healing, potentially due to high drug dose or presence of a high polymer load.
Polymers are added to DES systems to carry and control the drug distribution pharmacokinetics in the local tissue. The amount of polymer used in each DES varies in accordance to the amount of drug they are intended to load and influence the desired pharmacokinetics. Compared to other market available sirolimus-analogue DES, the Excella system carries a considerably lower dose of the anti-proliferative drug (~ 4.5 µg of novolimus/mm of stent length vs. ~7.5 µg of sirolimus/mm of Cypher™ 8 or 10 µg of zotarolimus/mm of Endeavor™ 9 or ~5.5 µg of Everolimus / mm of Xience™ / Promus™10). Consequently, a reduced amount of polymer is required to load the drug into the coronary vessel (the polymer thickness in this novel system is ~3 microns). Moreover, the Excella system has a methacrylate polymer, a material which has a long history of use in clinical applications and has already been proven to be biocompatible.
The 8-month in-stent late loss of 0.31 mm observed in the present study situates this novel device among the most effective DES systems. When compared to the published results of other DES tested in similar scenarios, the in-stent late loss of the Excella stent is lower than Endeavor™ (late loss ~0.6 mm)9,11, equivalent to Taxus™ (~0.3-0.4 mm)12,13 and slightly higher than Cypher™ and Xience™ / Promus™ (~ 0.1-0.2 mm)2,10,14.
The increase in late loss and neointimal tissue observed between the fourth and eighth month of invasive follow-up has been previously described for other DES systems studied with serial IVUS11 and may be explained by an ongoing endothelialisation process at four months.
Limitations
This study comprises a registry of only 15 patients with non-complex coronary lesions with nine-month clinical follow-up. The lack of a control group precludes direct comparison against bare-metal and/or other DES systems.
Conclusion
The next-generation Excella-novolimus eluting stent system was proven to be feasible, safe and elicited minimum neointimal proliferation at follow-up. Long-term data in more complex groups of patients should be considered to confirm its safety and efficacy profile.
Excerpt from a reviewer
The first-in-man study of the Excella stent features a new sirolimus analogue, novolimus. The study was well planned and thorough in its methodology. The principle finding of the study was a late lumen loss of 0.15 mm after four months and 0.31 mm after eight months, which is comparable to other commercially available drug-eluting stents (DES). However, a surprising finding was acute malapposition in six out of 15 patients (40%) despite the use of IVUS-guided implantation and high pressure deployment. The malapposition was persistent in five patients (33.3%). Lesions with heavy calcification were excluded from the study and although the stent platform is cobalt chromium (CoCr), the degree of malapposition raises questions about the structural properties of the stent platform and whether there is excessive acute recoil. Evaluation of a different CoCr DES revealed acute recoil of 4.3% (Tanimoto CCI 2007; 70(4): 515-523): with this comparative value, it would interesting to know the acute recoil for the Excella stent and whether this contributed to strut malapposition.

