Abstract
Aims: Radiofrequency renal artery denervation has been used effectively to treat resistant hypertension. However, comparison of lesion and thermodynamic characteristics for different systems has not been previously described. We aimed to assess spatiotemporal lesion growth and ablation characteristics of Symplicity and EnligHTN systems.
Methods and results: A total of 39 ablations were performed in a phantom renal artery model using Symplicity (n=17) and EnligHTN (n=22) systems. The phantom model consisted of a hollowed gel block surrounding a thermochromic liquid crystal (TLC) film, exhibiting temperature sensitivity of 50-78°C. Flow was simulated using 37°C normal saline with impedance equal to blood. Radiofrequency ablations with each system were delivered with direct electrode tip contact to the TLC. Lesion size was interpreted from the TLC as the maximum dimensions of the 51°C isotherm. Mean lesion depth was 3.82 mm±0.04 versus 3.44 mm±0.03 (p<0.001) for Symplicity and EnligHTN, respectively. Mean width was 7.17 mm±0.08 versus 6.23 mm±0.07 (p<0.001), respectively. With EnligHTN, steady state temperature was achieved 20 sec earlier, and was 15°C higher than Symplicity.
Conclusions: In this phantom model, Symplicity formed larger lesions compared to EnligHTN with lower catheter-tip temperature. The clinical significance of our findings needs to be explored further.
Introduction
Refractory hypertension affects about 10-30% of hypertensive patients1. Renal artery denervation has resulted in a significant and sustained blood pressure reduction in patients with resistant hypertension as demonstrated by clinical trials2,3. The procedure aims to interrupt the neurohormonal impulses arising from the kidneys, which have been implicated in the pathogenesis of essential hypertension4-7. Nerves responsible for these signals lie within the adventitia of the renal artery with about 90% of nerve fibres occurring within 2 mm from the intimal surface8. Endovascular denervation of the renal arteries has been performed through different energy modalities, including radiofrequency, cryoablation, ultrasound, and chemical denervation9-13. At present, the two most widely utilised systems are the radiofrequency Symplicity™ (Medtronic, Minneapolis, MN, USA) and EnligHTN (St. Jude Medical, St. Paul, MN, USA) systems. Radiofrequency energy causes tissue destruction by means of thermal injury14, when tissue temperature is raised above 45-50°C15. Both systems are optimised to operate with unique ablation settings summarised in Table 1.
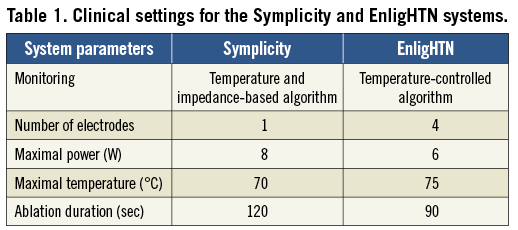
A predetermined algorithm is utilised for each system to ensure ablation safety. The goal is to achieve reasonably large lesions affecting the target nerves, whilst limiting the injury to the renal artery in order to minimise the risk of renal artery stenosis. It is therefore important to understand the basic biophysical properties for each system in relation to factors that may affect lesion formation. Assessment of the thermal characteristics of cardiac radiofrequency ablation using in vivo and in vitro models has been described previously16,17. Previous studies have demonstrated good correlation between radiofrequency ablation lesions performed in myocardium, and a Phytagel™ based myocardial phantom model incorporating a TLC film (LCR Hallcrest, Flintshire, UK)17. The study demonstrated the ability of TLC to map the dynamics of lesion formation, based on the 50°C isotherm, in high spatial and temporal resolution. Herein, we provide the first report to evaluate ablation characteristics for two renal denervation systems using a TLC phantom model of the human renal artery.
Methods
PHANTOM RENAL ARTERY MODEL
A renal artery model was prepared as per Chik17. Briefly, 1 gram of gel (Phytagel™; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in a mixture of distilled water and normal saline at a pre-specified concentration (70% distilled water and 30% normal saline). The mix was then brought to a temperature of 90°C, and poured into a well once cooled down to 75°C. The gel well was immersed in a tank that pumped saline at a constant temperature (37°C). Impedance of saline used in the tank was matched to that of blood at 37°C. To mimic the renal artery with a comparable diameter and physiological blood flow rate in a human renal artery18, specific modifications to the model were applied. In summary, this was achieved by pouring the gel around a 5 mm diameter metal rod holding a vertical TLC disc (colour bandwidth from 50-78°C) at the centre of the gel well. The rod was retrieved after gel cooling to form a hollowed tube in the centre of the gel simulating a renal artery lumen (Figure 1). The diameter of the phantom renal artery was kept constant (5 mm) for all gel blocks used in the experiment. A flow meter was used to maintain the rate of saline pumped into the phantom renal artery at 500 ml/min.
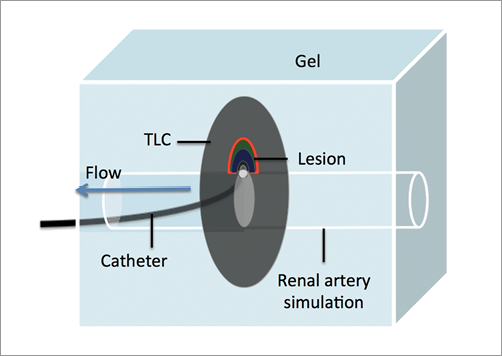
Figure 1. Schematic diagram of radiofrequency ablation in a renal artery phantom model.
RADIOFREQUENCY ABLATION ON TLC
An average of eight radiofrequency ablations per system were delivered on each gel block (n=3). A new TLC disc was used per gel block to avoid damage to the TLC by repeated heating. The order in which each system was tested was altered randomly with every new gel. To deliver radiofrequency ablations, the catheter (Symplicity or EnligHTN) was advanced through the phantom renal artery and positioned in plane with the TLC film. Contact was confirmed by direct visualisation, as the gel was transparent. Radiofrequency ablation was then applied to the gel using the recommended clinical parameters for each system as indicated in Table 1. In the case of EnligHTN, the small size basket was used to match the diameter of our phantom renal artery.
LESION MEASUREMENTS AND ANALYSIS
Photographs of the TLC colour gradient were taken at baseline and at 5 sec intervals during ablation using a digital camera (Canon EOS 5D Mark II; Canon Inc., Tokyo, Japan) and a light source (Canon Speedlite 580EX; Canon Inc.). In-house software was used to analyse the pictures and measure lesion dimensions at several ablation intervals (initially every 5 sec, then every 10 sec) as described previously17. Lesion size was defined using the 51°C isotherm. Lesion depth was measured from the gel electrode surface to the isotherm of interest (51°C). Lesion width was defined as the maximal width of the 51°C isotherm parallel to the electrode gel surface (Figure 2).
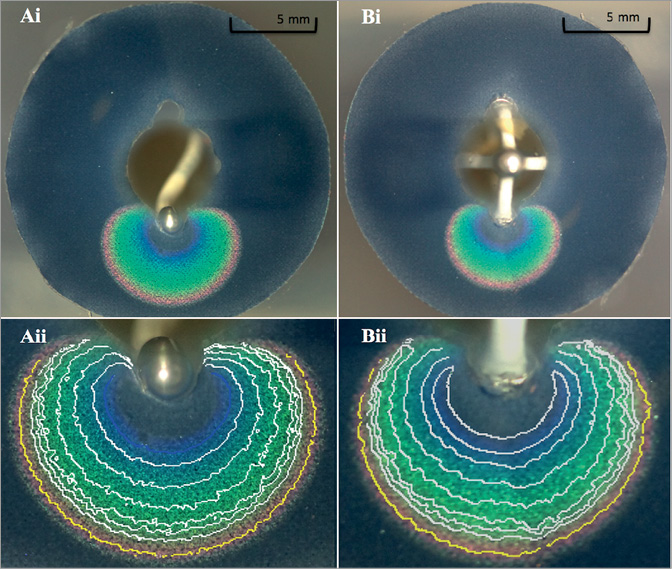
Figure 2. Coronal image of gel with Symplicity (Ai) and EnligHTN (Bi) radiofrequency ablation of TLC film with related isotherm gradient at 120 sec and 90 sec, respectively, and their magnifications (Aii & Bii). The isotherm of interest (51°C) is highlighted in yellow (Aii & Bii).
ELECTRODE SURFACE AREA IN CONTACT WITH GEL
For all ablations a cavity was created in the TLC and the gel to simulate endothelial tissue depression during electrode tip contact. The surface area in contact with the electrode was estimated to be about 50% of the total ablation electrode surface area for all energy applications. Measurements of electrode dimensions were carried out using a vernier scale (Figure 3).
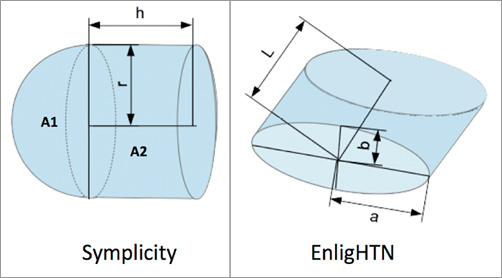
Figure 3. Schematic diagram of Symplicity and EnligHTN electrode tip measurements.
1. For Symplicity, total electrode surface area was calculated as follows:
Total surface area (ATS)=Surface area 1 (A1)+Surface area 2 (A2)
Given that r=0.62 mm and h=1.02 mm
A1=4 π r2 / 2=2.42 mm2
A2=2 π r h=3.97 mm2, hence,
ATS=6.39 mm2
Therefore, Symplicity electrode area in contact with gel=3.2 mm2.
2. For EnligHTN, total surface area (ATE)=2Lπ √ 0.5(a2+b2)=3.7 mm2, where L=0.97 mm, a=0.42, b=0.75 mm
Therefore, EnligHTN electrode area in contact with gel=1.85 mm2.
Statistical analysis
Based on preliminary data from gel 1 experiments, a total sample size of six ablations (three per catheter modality) was required to detect a mean difference of 0.66 mm for depth, and 1.54 mm for width with a power of 95% between each catheter modality when a=0.05 (two-tailed). As sufficient power was achieved to detect differences between modalities in the first experiment (gel 1), additional experimentation using multiple gels was performed to negate the effect of variation in the gel substrate, which may occur due to external factors (gel heating and solidification time). Mean depth and width for each system were determined using an unpaired two-tailed Student’s t-test. Each gel experiment for lesion dimensions (depth and width) was analysed using a two-way ANOVA. Pearson’s correlation was used to assess the relationship between lesion dimensions (depth and width) with time, and power with time. Statistics were performed on GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). A linear mixed effects model was used to calculate the rate of temperature rise over time for the two systems. S-PLUS Version 8 was used to fit linear mixed effects models to the catheter temperature. In these models, ablation repetition and gel were considered as random factorial effects and time as a random continuous variable. Catheter type, time (continuous) and catheter by time interaction term were considered as fixed effects.
Results
A total of 52 ablations were performed on the phantom renal artery model, comprising 26 ablations for each system. A total of eight ablations using Symplicity and four ablations using EnligHTN were excluded from the analysis due to incorrect plane alignment between the catheter tip and TLC plate. Incorrect plane alignment refers to a catheter positioned inadequately on the TLC surface, but which could still be in contact with the gel. Therefore, the centre of the ablation zone (where maximal lesion dimension could be measured) occurs either behind or in front of the TLC, which will subsequently lead to underestimation of lesion dimensions. The exclusion rate for the Symplicity catheter was much higher because catheter positioning was technically more difficult with the Symplicity catheter compared to EnligHTN. Radiofrequency ablation parameters are summarised in Table 2.
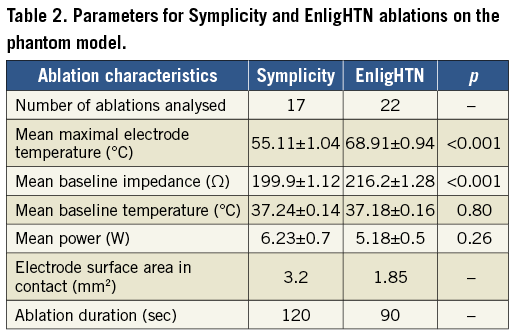
LESION DIMENSION
Mean lesion depth was 3.82 mm±0.04 and 3.44 mm±0.03 (p<0.001) for Symplicity and EnligHTN, respectively. Mean width was 7.17 mm±0.08 versus 6.23 mm±0.07 (p<0.001), respectively (Figure 4Ai, Figure 4Aii). Variability in lesion characteristics (range of width, and depth in mm) was observed between gels but, despite this, statistical significance between ablation systems was achieved (Figure 4Bi, Figure 4Bii). This variability could be attributed to minor differences in the gel and TLC set-up, as well as electrode-TLC contact.
When analysed at 90 sec, there was no significant difference in lesion depth between the Symplicity and EnligHTN, with mean depth of 3.51 mm±0.04 versus 3.44 mm±0.03 (p=0.16). However, at 90 sec the difference in lesion width remained significant with mean width of 6.76 mm±0.07 versus 6.23 mm±0.07 (p<0.001) for Symplicity and EnligHTN, respectively. During Symplicity ablation, lesions continued to grow gradually over time, whereas lesion growth appeared to trend towards a plateau with EnligHTN at an earlier time point than Symplicity (Figure 4Ci, Figure 4Cii).
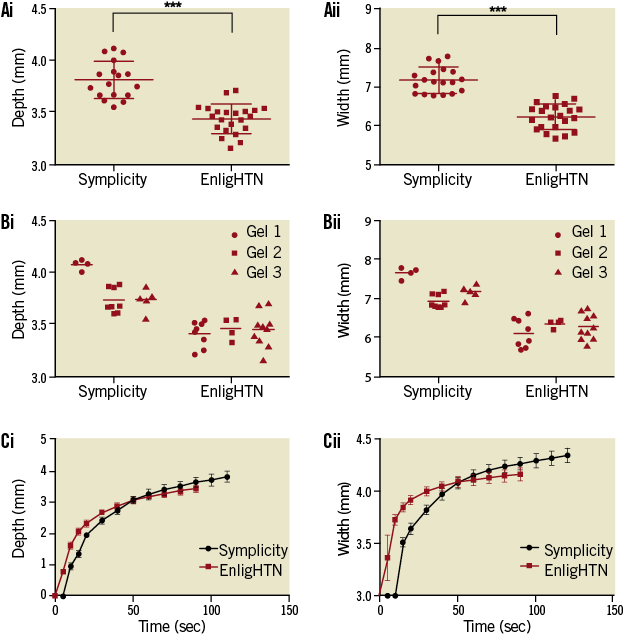
Figure 4. Scatter plots comparing lesion depth (Ai) and width (Aii) for Symplicity and EnligHTN for all three gels combined and each gel separately (Bi, Bii). Graph of lesion growth, depth (Ci) and width (Cii), over duration of radiofrequency application with both systems.
ABLATION THERMODYNAMICS
Using EnligHTN, steady state temperature was achieved within 20-30 sec, while Symplicity took longer (50 sec) (Figure 5A). This trend was consistent with the power output curve, whereby maximal power was reached within 10 sec for EnligHTN compared to a more gradual increase with Symplicity (Figure 5B). This delay in heating with the Symplicity system may be due to an automated algorithm used to increase ablation safety. The maximal power output limit for each system was reached with all ablations. Within the first 20 sec of ablation, the rate of Symplicity electrode temperature rise was 0.2°C per sec (SE 0.065) faster than EnligHTN (p<0.0024). Additionally, EnligHTN had a higher electrode temperature at steady state 68°C compared to 55°C for Symplicity, p<0.001.
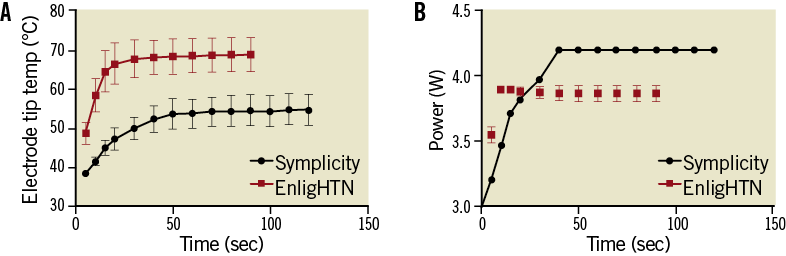
Figure 5. Graph showing temperature change (A) and power output (B) over the duration of radiofrequency application for Symplicity and EnligHTN.
Discussion
Renal artery denervation is a promising treatment option for patients with resistant hypertension. However, a lot of questions remain unanswered regarding this treatment approach, as has been outlined recently19. One of the unknown factors is the safety and efficacy across different ablation systems and energy modalities. The present study is the first to compare ablation characteristics of two commercially available radiofrequency renal artery denervation systems directly using a phantom renal artery model. We found that Symplicity created lesions that were significantly deeper and wider than EnligHTN when duration of radiofrequency energy delivery was 120 sec and 90 sec, respectively, as recommended by the manufacturers. When ablation time was capped at 90 sec, lesion depths were similar between systems but remained significantly wider using Symplicity.
Thermal necrosis of tissue using radiofrequency energy occurs as a result of resistive and conductive heating20. During radiofrequency ablation resistive heating occurs within 1 mm of the myocardial-electrode interface, whereas deeper tissue heating results from slower thermal conduction which occurs in a radial pattern away from the resistive zone. Factors that lead to a larger volume of resistive heating or higher current density at the electrode tissue interface will similarly increase the temperature radial gradient area and subsequently lesion size20. These include ablation power, ablation duration, electrode surface area in contact with tissue, and ablation temperature.
In general, power increase leads to a greater current density at the electrode tissue interface. Therefore, lesion size is proportional to ablation power. An in vivo closed chest endocardial ablation study by Wittkampf demonstrated an increase in lesion size with higher power settings21. Similar results were obtained during ex vivo ablations of a bovine left ventricle under superfusate flow (1 L/min)22. The amount of power delivered to the target tissue is however affected by tissue impedance and power dissipation into the blood pool, which varies between different positions within one patient and also between different patients. Both Symplicity and EnligHTN systems operate at low power settings compared to cardiac radiofrequency ablation generators and are regulated by a catheter tip-temperature and impedance feedback mechanism. In our phantom model, mean power delivered during radiofrequency ablation was comparable for both systems. Hence, differences in lesion dimensions between the two systems cannot be explained by differences in power output.
Lesion growth rate for radiofrequency ablation follows a monoexponential function with a rapid initial phase occurring locally to the area of resistive heating. Thereafter, growth reaches a steady state without subsequent increase in lesion size21,23. A study that compared cardiac lesion size at two ablation time points (60 and 120 sec), utilising low ablation power ranging from 20-50 W and superfusate flow, showed that larger lesions occurred at 120 sec compared to 60 sec22. Based on that study, we postulate that the relatively longer ablation time applied using Symplicity was a significant factor in the observed increase in lesion size compared to EnligHTN. In support of this, neither system reached growth steady state, which is probably due to the high flow rate accounting for dissipation of power and reduction in power delivered to the tissue. This suggests that longer duration of ablation could potentially result in larger lesion size with both systems.
Lesion size is also affected by electrode tissue interface area. Haines described a linear relationship between electrode radius and lesion radius under constant electrode tissue interface temperature using a thermodynamic model. His model was validated using perfused and superfused canine right ventricular free wall24. Larger electrode radius formed bigger lesions when power density and tissue interface temperature remained constant. Nevertheless, higher overall power was required to maintain a similar power density. Additionally, lesion dimensions increase with increasing electrode length provided contact is adequate25,26. It has been demonstrated that increases in electrode tissue interface area and area exposed to convective cooling by blood are both responsible for increase in lesion size with larger electrodes27. Thus, the larger Symplicity electrode tissue interface area may have contributed to the difference in lesion size between the two catheters. Of note, the Symplicity system is based on a temperature and impedance algorithm, while the EnligHTN system has a temperature-controlled algorithm. The difference in system operation could also influence lesion size.
During temperature-controlled radiofrequency ablation, lesion size is directly proportional to electrode tissue interface temperature20. Temperature monitoring devices (thermocouple or thermistor) embedded within the ablation electrode monitor electrode tip temperature during radiofrequency ablation. However, a discrepancy between catheter tip temperature and tissue temperature can occur due to various factors including convective cooling by blood or irrigation. Whilst EnligHTN ablations reached higher electrode tip temperature compared to Symplicity for all ablations, Symplicity lesions were larger. This is probably due to the contribution of other factors discussed above affecting lesion size. Moreover, temperatures at the TLC electrode interface for all ablations were higher than those detectable by the TLC sheet (indicated by colour clearing, Figure 2), suggesting that the temperature measured by the electrode tip during renal artery ablation was lower than that of the electrode tissue interface. This difference was probably due to cooling of the electrode tip by high flow rate in the renal artery.
Thrombus formation and intimal disruption have been reported in a study using optical coherence tomography during renal artery denervation, more frequently with EnligHTN28. It is unclear if this is due to the higher temperature achieved during ablation and more rapid temperature increase with EnligHTN observed in our study.
Clinical implications
With numerous emerging technologies and catheter designs for renal artery denervation, it is critical to understand the basic biophysics of radiofrequency ablation for each device and the affect of that design on lesion formation. Moreover, identifying the unique characteristics of each renal denervation system is of pivotal importance in order to optimise ablation safety and efficacy. Each system needs to be evaluated further in clinical trials to establish its clinical safety and efficacy.
In the present study, adequate contact between the catheter tip and the TLC was confirmed by direct visualisation. However, in clinical practice this is difficult to establish and maintain, especially with the single electrode Symplicity catheter. This is important, as adequate electrode tissue contact is imperative for lesion formation29. In addition, despite the statistical difference in lesion size, it is difficult to anticipate the clinical significance of this finding.
Study limitations
The phantom renal artery used for our study does not replicate the heterogeneity of renal arterial layers, which have unique conduction properties and are also surrounded by fat. This may consequently affect the distribution of thermal injury. Nonetheless, the two systems were compared under an identical controlled environment with no confounding variables. This model also has the advantage of allowing the evaluation of temporal progression of lesions, which is difficult to assess in vivo. To allow for the comparison between the two systems, the flow rate and the renal artery diameter were kept constant; therefore, the effect of alteration in flow rate, arterial spasms and vessel wall oedema that can occur during the application of radiofrequency energy could not be simulated. In addition, EnligHTN lesions were more consistent in size compared to Symplicity lesions, as seen in Figure 4B. This could be as a result of variations in the electrode contact area with the gel-TLC surface, which were carefully placed but were more difficult to establish between the Symplicity experiments. Evaluation of collateral damage using the phantom model is not possible; however, a preclinical safety trial conducted with the Symplicity system reported no collateral damage to kidneys or nearby structures30. Finally, the TLC film used in the phantom had a colour change sensitivity range between 50-78°C; therefore, we were unable to detect temperatures beyond this range.
Conclusion
Lesion size was larger for the Symplicity renal artery denervation system compared to EnligHTN when radiofrequency ablation was performed on a phantom renal artery model. The difference in lesion size was more pronounced for lesion width, with a smaller difference in lesion depth. This was achieved with a gradual increase in temperature and lower electrode tip temperature at steady state with the Symplicity system. It is likely that the larger electrode surface area and longer ablation duration with Symplicity could have accounted for the difference in lesion size.
| Impact on daily practice One important criterion for successful renal artery denervation is the delivery of safe and effective ablation lesions. Currently, different systems are being used to deliver these ablations in patients with resistant hypertension. Understanding the biophysical properties of the systems and the differences in lesions formed is essential to improve the techniques and clinical outcome. The renal artery phantom model described here provided a platform to identify lesion properties from two different systems under specific conditions. This model can be applied to available and future renal denervation systems, thereby providing a preclinical simulation that would objectively inform the clinical parameters for the lesions created. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

