Abstract
Aims: Recent studies of drug-eluting stents for unprotected left main coronary artery (LMCA) disease have been encouraging. We examined the performance of sirolimus-eluting stents (SES) for this indication.
Methods and results: This retrospective study included 228 consecutive patients (mean age=68±11 years, 80.6% men, 26.3% diabetics) who underwent implantation of SES for de novo LMCA stenoses. The mean additive and logistic EuroSCOREs were 5.2±3.9 and 8.2±13.2, respectively. The main objective of this study was to measure the rate of major adverse cardiac events (MACE), including death, myocardial infarction and target lesion revascularisation (TLR) at 12 months. Other objectives were to measure the rates of in-hospital MACE and 12-month TLR. Outcomes in 143 patients with (BIF+ group), versus 84 patients without (BIF– group) involvement of the bifurcation were compared. The pre-procedural percent diameter stenosis (%DS) was 60.1±11.2 in the BIF+ versus 54.7±12.2% in the BIF- group (p=0.008), and decreased to 18.0±9.7 and 13.9±11.3%, respectively (ns), after SES implant. The overall in-hospital MACE rate was 3.5%, and similar in both subgroups. The 1-year MACE rate was 14.5% overall, 16.8% in the BIF+ and 10.7% in the BIF– subgroup (ns).
Conclusions: SES implants in high-risk patients with LMCA stenoses were associated with a low 1-year MACE rate. Stenting of the bifurcation was associated with significant increases in neither mortality nor 1-year MACE rate.
Introduction
Left main coronary artery (LMCA) disease is found in approximately 3 to 5% of patients who present with chronic angina pectoris. It is classified as protected when > 1 patent graft to the left coronary circulation is present, and unprotected in absence of a patent graft. The short length of the LMCA, and the often severely calcified lesions, which may extend into the two main bifurcating arteries, represent, in unprotected disease, sources of major or fatal complications, in the periprocedural period and on the long term. Indeed, early attempts at balloon angioplasty (with or without stent implantation), were associated with adverse intermediate and long-term outcomes, even after apparently successful procedures1,2. In absence of controlled studies showing a survival advantage conferred by PCI in this subset of patients, coronary artery bypass surgery has remained the main form of treatment of LMCA stenoses for over 30 years3,4.
Major technological advances in PCI have been achieved in the past decade, including the nearly systematic use of coronary stents and of effective antithrombotic regimens, stimulating a renewed interest in the percutaneous management of LMCA disease. Initial results from single centres in limited numbers of patients have been encouraging. In particular, data published recently have confirmed that stenting of unprotected LMCA can be performed with very high procedural success rates5-8. As is the case with other coronary vessels, the development of restenosis represents the main factor limiting the long-term success of PCI for unprotected LMCA. In a recent study, the angiographic restenosis rate was closely related to the size of the reference vessel diameter9. Distal LMCA and bifurcation lesions have also been identified as important predictors of target lesion revascularisation (TLR)10. It is noteworthy that the rates of in-stent restenosis following PCI for LMCA disease, using bare metal stents (BMS), are generally lower than with other lesion subsets, perhaps because of the larger vessel size and the insistence of operators on achieving excellent angiographic results. They remain, however, as high as 30% in patients at particularly high risk.
This study examined the outcomes of PCI using sirolimus-eluting stents (SES) in patients with unprotected LMCA disease, with a focus on the presence versus absence of involvement of the LMCA bifurcation.
Patient population and methods
This retrospective study (Retrospective Left Main Registry) was reviewed and approved by the Ethics Committee of all participating medical centres, and all patients granted their informed consent to include their data in this retrospective analysis. Efforts were made to obtain the consent from relative of patients who had died before the beginning of recruitment. The study was designed to examine the safety and effectiveness of the CypherTM and Cypher SelectTM SES (Cordis Corporation, Miami Lakes, FL, USA) in patients who, between April 2002 and May 2004, underwent PCI and stent implantation for de novo, unprotected LMCA stenoses, i.e. without a patent surgical graft bypassing the LMCA. They were eligible for inclusion into the study if the target lesion was located in the LMCA, including the adjacent 1-cm segments of proximal left anterior descending (LAD) or left circumflex (LCX) artery. Therefore, a procedure was considered to be a LMCA intervention when stenting involved the main vessel, including when the main part of the stent(s) extended into the LAD or LCX arteries. Patients were excluded from the analysis if 1) another type of stent was implanted into the target lesion together with an SES during the index procedure, 2) the stent was implanted for acute myocardial infarction (MI), or cardiogenic shock, or any other unplanned (bail-out) LMCA stenting, 3) a stent of appropriate size was not available (overexpansion of the stent, defined as a > 0.5 mm difference between stent and vessel diameters, was not allowed), and 4) the estimated life expectancy of the patient was <18 months at the time of the index procedure.
The 14 medical centres (appendix) who contributed patients to this study were chosen on the basis of a large volume of PCI performed and proven experience with stenting of unprotected LMCA stenoses. The investigators participating in the registry were in charge of the preliminary selection of the patients, based on the pre-specified inclusion/exclusion criteria. In a second step, and based on an independent review of the baseline angiograms, a few patients were excluded by the core laboratory (Figure 1).
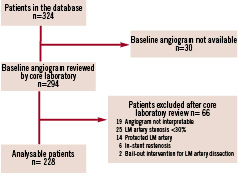
Figure 1. Patient screening and enrolment flow chart.
Main study objectives
The main objective of the study was to measure the rate of major adverse cardiac events (MACE) at 12 months after the index procedure. MACE was defined as death, Q wave or non-Q wave MI, emergent coronary artery bypass surgery, or repeat percutaneous TLR. Other study objectives were to measure 1) in-hospital MACE rate, and 2) stent thrombosis rate up to 12 months after the index procedure.
Periprocedural data collection
The collection of data for this registry was based on a review of available records.
Pre-procedure. All procedures were conducted according to local clinical practices. Whenever available, the following information was recorded from baseline observations: 1) Patient demographic and clinical characteristics, including assessment of angina status and myocardial ischemia, 2) additive and logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE)11,12, 3) screening laboratory tests, including serum creatinine measured within seven days prior to the index procedure, 4) serum creatine kinase, creatine kinase-MB and troponin I or T, measured within 24 h of the index procedure, and 5) 12-lead electrocardiogram (ECG) recorded within 24 h prior to the procedure.
Stent implantation procedure. Stents between 8 and 33 mm in length and 2.25 and 3.50 mm in diameter were implanted according to the standard practices and procedures of each participating hospital. Direct stenting, use of multiple stents and treatments of bifurcation were allowed. Information regarding the index lesion and its stenting procedure and techniques (single stent, crush, V- or T-stenting, kissing balloon, culotte) was recorded, as well as information regarding other lesions that might have been treated during the index procedure. Detailed data on complications and their management were collected when a device failure had occurred. Procedural failure was defined as 1) failure to achieve a <50% diameter stenosis by quantitative coronary angiography (QCA) using any percutaneous method, or 2) in-hospital death, MI, or repeat revascularisation of the target vessel.
Post-procedure. The procedure was considered complete after removal of the guiding catheter from the patient. When a guiding catheter was re-introduced, it was considered a repeat intervention. Enzymes were measured when clinically indicated, according to standard medical practices. A 12-lead ECG was recorded and serum creatinine concentration measured within 24 h after the procedure or at the time of discharge of the patient from the hospital.
Definitions of stent thrombosis and antithrombotic therapy
Death not attributable to a non-cardiac cause, Q-wave MI, or vascular occlusion requiring revascularisation, each occurring within 30 days after the index procedure, were considered due to stent thrombosis. Stent thrombosis was also diagnosed when a thrombus was found within the stented vessel at the time of angiography obtained for clinical manifestations consistent with myocardial ischaemia. Late stent thrombosis was diagnosed when an MI occurred in the territory of the target vessel, with angiographic documentation of a thrombus or total occlusion at the site of target revascularisation, > 30 days after the index procedure, in absence of interim revascularisation of the target vessel.
Antithrombotic therapy was administered according to the clinical practices of the participating medical centres. The following regimen was generally administered:
Pre-procedural. Aspirin, 100-325 mg, starting within 24 h prior to the procedure, clopidogrel in a loading dose of 300-600 mg within 24 h before or after the procedure, followed by 75 mg once or twice daily, or ticlopidine, in a loading dose of 500 mg within 24 h before or after the procedure.
Intra-procedural. Heparin, in an initial intravenous bolus, with additional boluses, as necessary, to maintain an activated clotting time > 250 sec (> 200 sec in presence of a glycoprotein IIb/IIIa inhibitor).
Post-procedural. Clopidogrel, 75 mg daily or twice daily, or ticlopidine, 250 mg bid, for 2-12 months, and aspirin 100-325 mg daily, indefinitely.
Quantitative coronary angiography
All angiograms underwent QCA analysis by an independent core laboratory (Bio-Imaging Technologies, B.V., Leiden, The Netherlands), using the QCA-CMS version 6.0 analytical software program (Medis Medical Imaging Systems B.V., Leiden, The Netherlands), for the estimation of TIMI flow and description of lesion characteristics, including length, classification according to the American Heart Association/American College of Cardiology, eccentricity, dissection grade, and presence of calcification or thrombus. Angiograms, performed at 9±3 months of follow-up, were available in 44 patients (19.3%). Therefore, this report, which reflects “real world” medical practices, does not include a detailed analysis of follow-up angiographic data. Unscheduled angiograms performed for investigations of new symptoms consistent with myocardial ischaemia or for abnormal exercise testing were reviewed by the Clinical Events Committee (CEC).
The pre-procedural percent diameter stenosis (%DS) was calculated as (1-Dobstr/Dref) x 100%, in which Dobstr is the baseline minimum luminal diameter, and Dref is the user-defined post-procedural reference diameter. Because of the lesions complexity and the morphological and technical constraints that might limit an optimal visualisation of the LMCA together with the LCX or LAD, without foreshortening or overlap, the entire stented area was analysed by QCA if either the LMCA and LCX, or the LMCA and LAD, were stented without gaps. The reference diameter used for the analysis was software-interpolated or user-defined. In addition, after the procedure, a user-defined reference diameter was used
to calculate the baseline %DS.
Qualitative analysis
A qualitative analysis of the target vessel was performed according to Yamane et al13. In case of >1 stenosis in the stented area, this analysis was limited to the segment with the highest %DS.
Long-term patient follow-up, study monitoring, data collection and management
Patients were followed by each enrolling centre, according to the local clinical practices. Data available in medical records were used to determine the patient’s clinical outcome up to one year. At each visit, historical information pertaining to the patient’s general health was gathered, with special attention to complaints consistent with recurrent angina, possible MACE, concomitant drug regimen and cardiac interventional treatment that might have been performed since the previous contact. A physical examination was performed and a 12-lead ECG was recorded. Interventions and events in response to angiograms performed during follow-up were obtained, including percutaneous or surgical coronary revascularisation procedures, procedural characteristics, need for re-hospitalisation, days in intensive care, overall duration of hospitalisation, and prescription of cardioactive medications.
Baseline and follow-up data were monitored and compiled at regular intervals by the study sponsor, or a designee, who verified their accuracy by comparing the information entered onto case report forms with that contained in the source documents, with special attention to the precise reporting and documentation of interim procedures and adverse clinical events. The data were transferred via an Internet-based data capture and reporting system organisation (Phase Forward Europe Ltd. Berkshire, United Kingdom), and managed and statistically treated by an independent clinical research (CTO, Clinquest Europe B.V., Oss, The Netherlands).
Clinical events committee The members of the CEC were interventional cardiologists who did not participate in the trial, and were not associated with Cordis. The CEC developed the specific criteria used for the classification of major clinical events. The members of the CEC, who were unaware of the results of the study, met regularly to review and adjudicate all MACE, including types of MI and deaths, based on the review of ECG, results of laboratory testing, narrative contained in medical records, and all other pertinent information available. In addition, putative relationships between adverse clinical events and devices, procedures or medications were thoroughly scrutinised.
Statistical analysis
All analyses were based on the intention-to-treat principle. Descriptive statistics for continuous variables are presented as means±standard deviation (SD) and, where appropriate, as ranges and differences with 95% confidence intervals (CI). Categorical variables are presented as absolute numbers (n) and relative frequency (%). Based on the Yamane LMCA classification13, we distinguished two morphology categories: 1) bifurcation involved (Yamane morphology is distal or diffuse), and 2) no bifurcation involved (Yamane morphology is ostial or body). Clinical events, including death, MI, and revascularisation are reported on a per patient basis. Between-groups differences were examined by Fisher’s exact test for categorical variables and two-sample t-test or Wilcoxon signed rank test for continuous variables. Survival free from MACE and TLR during the 1-year of follow-up were analysed using the Kaplan-Meier method. The statistical significance of differences between event-free survival curves was examined with the use of the log-rank test. Multiple variable Cox regression analysis was used to adjust for possible confounders and examine the prognostic value of several variables, including age, lesion length, and history of PCI and diabetes. Hazard ratio and 95% CI were calculated.
A two-sided p value <0.05 was considered statistically significant. The SAS(r) version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Study population
The screening and enrolment of the 228 patients who fulfilled the study inclusion and exclusion criteria is summarised in Figure 1. Their mean age was 68±11 years (range: 37-90), 80.6% were men, and 26.3% were diabetics. The mean additive and logistic EuroSCOREs were 5.2±3.9 (n=171) and 8.2±13.2 (n=183), respectively. Additional baseline clinical characteristics are presented in Table 1.
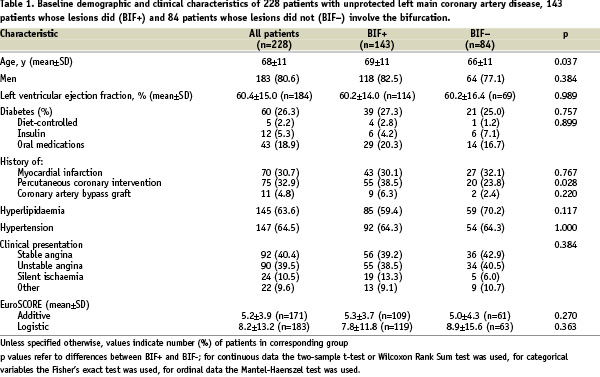
The bifurcation was involved in the lesion of 143 patients (BIF+ group), and not involved in 84 patients (BIF- group). For one patient this information was not available.
Results of creatine kinase-MB blood concentrations measurements were available before PCI, and between six and 8 h, 12 and 16 h, and at 24 h or at the time of discharge from the hospital in 99 (44%), 77 (34%), 51 (22%) and 51 (22%) patients, respectively.
Left main coronary artery and lesion characteristics
The LMCA and its lesion characteristics are summarised in Table 2.
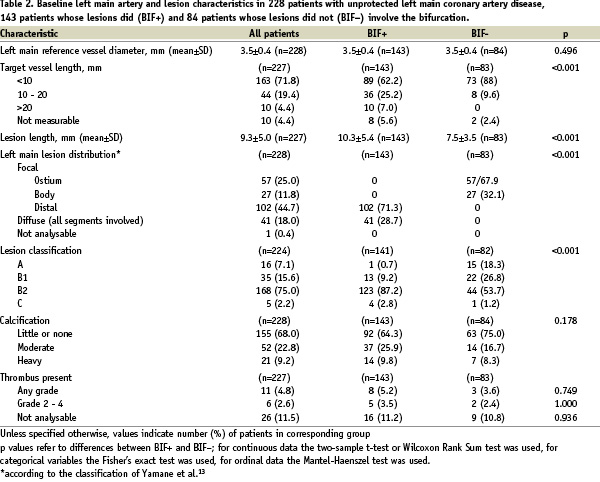
The mean lesion length was 9.3±5.0 mm. One fourth of the lesions were ostial, 75% were class B2 lesions, and moderate or heavy calcifications were present in 1/3 of patients, while thrombi were rarely present. Among the 143 patients included in the BIF+ group, 102 had focal disease of the distal LMCA, and 41 patients had diffuse disease.
Index procedure characteristics and immediate angiographic outcomes
The mean length of the 310 stents that were implanted in the 228 patients was 14.9±7.3 mm (range 8 - 33), and mean diameter 3.3±0.3 mm (range 2.25 - 3.5). Stents between 8 and 18 mm in length were implanted in 81.5%, and between 3 and 3.5 mm in diameter in 90.6% of patients. Procedural details and immediate angiographic outcomes for the entire population and for each study group are shown in Table 3.
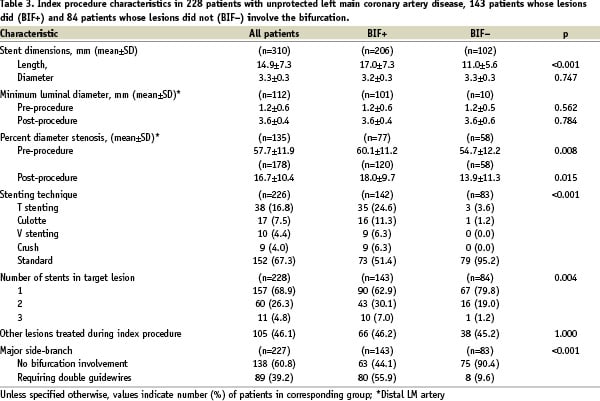
As expected, the BIF+ group received a greater number and longer stents than the BIF- group. The pre-procedural %DS was significantly greater in the BIF+ than in the BIF- group. While a marked reduction in %DS was achieved in both groups, the post-procedural %DS remained significantly greater in the BIF+ group (Table 3).
Clinical outcomes
In-hospital. The rates of death, MI and TLR that occurred during the index hospitalisation in the overall study population and in each study subgroup are shown in Table 4A. The overall rate of MACE was 3.5%, including 1.8% rate of death and 2.2% rate of MI. There was no difference in the short-term MACE rates between the BIF+ and BIF– groups. A single patient, in the BIF+ group, suffered a non-fatal stent thrombosis.
One-year. The 1-year rates of death, MI and TLR for the entire population and for each study group are shown in table 4B.
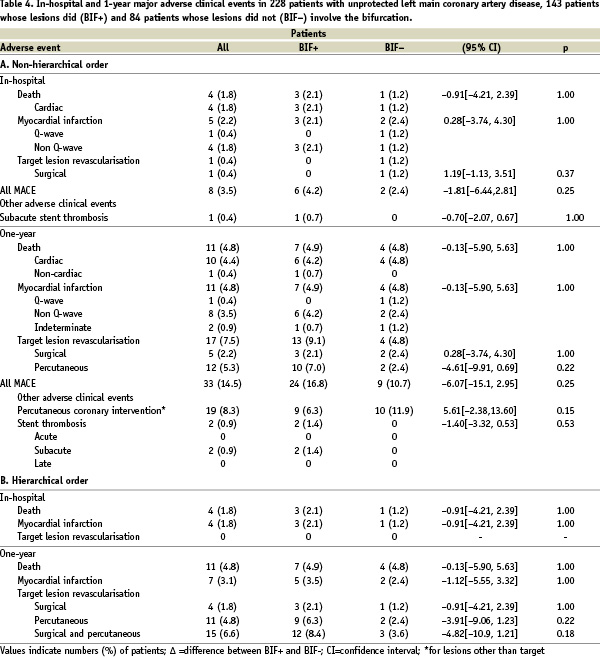
The overall rate of MACE was 14.5%, including 4.8% rate of death, 4.8% rate of MI, and 7.5% TLR. The overall rate of MACE was higher in the BIF+ (16.8%) than the BIF- (10.7%) group, though the difference was not statistically significant (Figure 2).
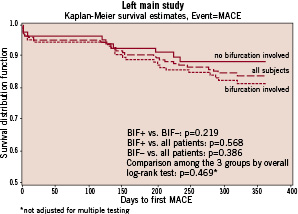
Figure 2. MACE-free 1-year survival in the overall population and in patients whose LM lesion did versus did not involve the bifurcation.
Similarly, no difference was observed between the two groups in survival free from TLR (Figure 3).
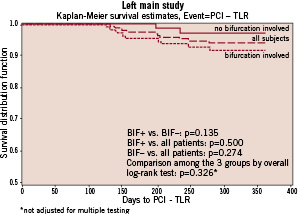
Figure 3. TLR-free 1-year survivals in the overall population and in patients whose LM lesion did versus did not involve the bifurcation.
The cumulative 1-year rate of non-fatal stent thrombosis was 1.4% in the BIF+ and 0% in the BIF- group. By multiple variable Cox regression analysis, after adjustment for age, lesion length, history of PCI and diabetes, the likelihood of MACE was 1.98-fold higher (95% CI 0.86, 4.56; p=0.11), and the likelihood of TLR 3.4-fold higher (95% CI 0.72, 16.27; p=0.12) in the BIF+ than the BIF- group.
Discussion
The main conclusions drawn from this analysis are: 1) angioplasty of the LMCA associated with placement of SES in a high-risk population was associated with a low overall 1-year rate of MACE, and 2) stenting of the LMCA bifurcation was associated with neither an excess mortality nor a significant increase in rates of MACE or TLR at one year of follow-up.
Low 1-year rates of major adverse cardiac events
The management of LMCA trunk lesions, previously limited to surgeons, is now accomplished by implanting drug-eluting stents (DES)5-8, with a view to eliminate complications1,14 or unacceptable rates of restenosis associated with BMS15-17. Registries and randomised studies are currently in progress to assess more precisely the risks and benefits of LM trunk angioplasty and DES implantation. The present registry of 228 high-risk patients, strictly selected among 324 patients screened by several investigators, is a valuable update on the use of SES for this indication. Indeed, the 228 patients, whose mean age was 68±11 years (26% diabetics), had a high EuroSCORE. Despite these high-risk characteristics, their in-hospital and 1-year mortality was limited to 1.8% and 4.8%, respectively. Some retrospective studies of surgical revascularisation for LMCA stenoses have reported rates of in-hospital mortality between 2 and 7%, and between 6 and 14% at one year18-20. Reports of smaller series of patients treated electively confirm a low in-hospital mortality and 1-year survival between 97 and 100%5-7. These mortality rates are similar to those observed in historical series of patients treated with BMS15,16,21.
Furthermore, the 7.5% overall rate of repeat TLR is undoubtedly attributable to the use of SES, in contrast with the approximately 20% 1-year rate of TLR achieved with BMS15,16,21. These improved results obtained with SES confirm those observed in the RAVEL and SIRIUS randomised trials22-23.
Sirolimus-eluting stents and left main coronary artery bifurcation
Among the 228 patients included in the registry, 143 presented with distal LMCA disease requiring treatment of the bifurcation. We found no significant difference in in-hospital mortality between patients with (2.1%) versus without (1.2%) treated bifurcations, reflecting the very high technical skills of the operators who performed these procedures. Furthermore, the 1-year mortality was nearly identical (4.9% with versus 4.8% without bifurcation lesions) in both groups and, by multiple variable analysis, no significant difference was observed at one year with respect to the incidence of MACE. Likewise, while a trend was observed toward a higher rate of TLR with treatment of LMCA disease involving the LAD-CFX arteries bifurcation (9.1%) than with lesions not involving the bifurcation (4.8%), the difference was not statistically significant. By multivariate analysis, after adjustments for multiple variables, bifurcation lesion was not an independent predictor of MACE or TLR. In contrast, in a study of 130 patients, of whom 94 had bifurcation lesions, Valgimigli et al found that involvement of the distal trunk was a major predictor of adverse outcomes24. That study included SES as well as paclitaxel-eluting stents (PES), raising the issue of a possible difference in outcomes associated with each drug. However, the recent publication of two studies that yielded conflicting results precludes the drawing of definitive conclusions. The first is a retrospective analysis derived from the RESEARCH and T-SEARCH registries, including 55 patients treated with SES and 55 with PES. The clinical characteristics of the two groups were similar and, at two years, the rates of MACE were similar in both groups (25% for SES versus 29% for PES)25. The second report is a randomised comparison of 103 SES versus 102 PES. While mortality rates were similar in both groups, a significant difference (p<0.05) in TLR was observed in favour of SES (4%) versus PES (13%)26. The multiple techniques used, including T-stenting, V-stenting, Y-stenting, culotte, and anatomical reconstruction, with or without kissing balloon following the stent deployments might explain these discordant results. A uniform treatment will probably have to await the availability of a bifurcated DES dedicated to the management of distal LMCA lesions. In our study, the relatively small number of patients included in each subgroup, and the follow-up analysis limited to one year, are additional factors that might explain the absence of significant differences in outcomes observed between BIF+ and BIF– patients. The distinctly higher a) in-hospital mortality, b) rate of MI at one year, and c) need for re-intervention at one year in the BIF+ than in the BIF– group suggest that an analysis of a larger sample population, observed for a longer period, would have shown a significantly higher MACE rate in patients whose treatment included the bifurcation.
Long-term outlook of drug-eluting stents in left main coronary artery disease
The 1-year results, which allowed the commercial release of DES seem now insufficient to satisfy the cardiology community27. Follow-ups of large patient populations for 3-5 years are needed to make definitive decisions with regard to the long-term acceptance of this form of treatment. Because of its prognostically sensitive localisation, stenting of the LMCA is in need of particularly meticulous long-term scrutiny. Instead of drawing assertive and definitive conclusions28, it might be preferable to await the results of ongoing randomised trials (SYNTAX, COMBAT). Furthermore, the prescription of optimal antiplatelet therapy, which includes the monitoring of clopidogrel and aspirin (the latter particularly after the withdrawal of clopidogrel), is the undisputed cornerstone of treatment with DES, as strongly suggested by the recently published analysis of data from a North American registry29.
Limitations of our study
There are four important limitations of our study, including 1) its retrospective design 2) the incomplete angiographic data, with a rate of long-term angiographic follow-up limited to <20% of patients, reflecting the variability of practices among investigators, who did not always deem appropriate to proceed with routine angiography in patients whose non-invasive assessment of myocardial ischemia was negative, 3) our inability to precisely determine the duration of dual antiplatelet therapy with clopidogrel and aspirin, and 4) as mentioned earlier, the relatively small sample population and follow-up limited to one year, which may have precluded the demonstration of statistically significant differences in outcomes between patients with, versus without bifurcation lesions.
Conclusions
While the relative safety and efficacy of percutaneous versus surgical management of LMCA lesions remain to be determined in randomised studies, our observations confirm the feasibility and encouraging results of PCI described in previous reports. It is, in any case, the only option in patients who are not surgical candidates.
Acknowledgements
The authors acknowledge the support of Cordis Corporation Inc., Clinical Research Europe, in the design of the study, and in the data collection and analysis.
Appendix
Co-principal investigators: Jean Fajadet, MD, Clinique Pasteur, Toulouse, France; Paul Barragan, MD, Polyclinique les Fleurs, Ollioules, France.
Co-investigators and participating institutions: Imad Sheiban, MD, San Giovanni Battista Hospital, Torino, Italy; Patrick Serruys, MD, Erasmus Medical Center, Rotterdam, the Netherlands; Antonio Colombo, MD, Columbus Hospital, Milan, Italy; Ricardo Seabra-Gomes, MD, Hospital Santa Cruz, Lisboa, Portugal; Jean-Jacques Goy, MD, Clinique Cecil, Lausanne, Switzerland; Stéphane Cook, MD, University Hospital, Bern, Switzerland; Paolo Rubino, MD, Montevergine Clinic, Mercogliano, Avellino, Italy; Thierry Lefèvre, MD, Institut Hospitalier Jacques Cartier, Massy, France; Dariusz Dudek, MD, Jagiellonian University Medical College, Kraków, Poland; Ferdinand Kiemeneij, MD, Onze Lieve Vrouw Gasthuis, Amsterdam, The Netherlands; Corrado Tamburino, MD, Ferrarotto Hospital, Catania, Italy; Joachim Schofer, MD, Center for Cardiology and Vascular Interventions, Hamburg, Germany.
Clinical Events Committee: Christian Spaulding, Hopital Cochin, France; Victor Legrand, CHU Liège, Belgium.

