Abstract
Background: The randomised Stenting Coronary Arteries in Non-stress/benestent Disease (SCANDSTENT) trial reported considerably less angiographic restenosis after implantation of sirolimus-eluting stents (SES) vs bare metal stents (BMS) in patients with complex coronary lesions. The purpose of this study was to evaluate the clinical outcome after a majority of the SCANDSTENT patients had stopped the dual antiplatelet therapy.
Methods and results: The SCANDSTENT trial randomly assigned 322 patients with symptomatic complex coronary artery disease (occlusions, bifurcations, ostial or angulated lesions) to receive SES or BMS. At 15 months after stent implantation, when 80% of the patients had stopped taking clopidogrel, six patients in the SES group and 10 in the BMS group had died or suffered a myocardial infarction (non significant [NS]). Compared with BMS, SES reduced the rate of target vessel revascularisation (TVR) from 33.1% to 5.6% (P<0.001) and the frequency of major adverse cardiac events from 35.7% to 8.6% (p<0.001). Definite stent thrombosis was observed in five patients in the BMS group, and two cases of probable and possible stent thrombosis were observed in the SES group (NS). One case of possible SES thrombosis occurred more than one year after stent implantation.
Conclusions: Compared with BMS, SES markedly reduced the frequency of TVR and MACE within 15 months in SCANDSTENT patients with complex coronary artery lesions without development of delayed restenosis.
Introduction
Drug eluting stents reduce the rate of clinical restenosis in simple coronary artery lesions1-5, whereas the experience of their use in complex lesions is limited6-9. The authorities have been reluctant to recommend the use of drug eluting stents in certain coronary lesions and advise waiting further investigations, before recommendations are made to implant drug-eluting stents in complex coronary lesions such as ostial lesions, total occlusions and bifurcations10.
The SCANDSTENT Trial was conducted to evaluate the clinical and angiographic outcome after implantation of one or more sirolimus-eluting stents (SES) or bare metal stents (BMS) in patients with complex coronary artery lesions. We have earlier reported the angiographic results of the SES versus BMS implantation at six months11.
There has recently been some concern about development of late adverse cardiac events including stent thrombosis in drug eluting stents, especially in the period after patients discontinue their dual antiplatelet medication12-14. This report focuses upon the clinical effect of implanting SES versus BMS in complex coronary artery lesions extending the observation period beyond the point of changing dual to mono-antiplatelet therapy using recently proposed revised definitions of stent thrombosis15.
Methods
Study design
The main purpose of this study was to compare the clinical outcome 15 months after randomised implantation of either SES or BMS for treatment of patients with complex coronary artery lesions. Criteria for inclusion and exclusion of patients have been described elsewhere11. Briefly, patients with symptomatic coronary artery disease and at least one complex lesion in a native coronary vessel were included provided they had at least one total or subtotal occlusion with a length of > 15 mm, a bifurcation lesion with a side branch > 1.75 mm in diameter, a lesion located within 5 mm of an ostium or located in an angulation > 45 degrees within the lesion. Important exclusion criteria were myocardial infarction < 3 days before the procedure, lesions located in unprotected left main stems or in bypass grafts. The protocol was approved by the local ethics committees of the participating hospitals, and all patients consented in writing to participate.
Randomisation and procedures
Randomisation was performed by computerised assignment stratified with regard to sex and diabetes. The lesions were treated by standard percutaneous interventional methods avoiding debulking techniques. The BMS Bx Velocity stent mounted on the balloon expandable delivery system, Sonic (Cordis/Johnson & Johnson, USA) or the SES Bx Velocity stent with sirolimus eluting properties, Cypher (Cordis/Johnson & Johnson, USA), were implanted in the lesions under high pressure (> 12 atmospheres). Implantation of more than one stent was allowed to cover the entire lesion, and side branch stent implantation was performed at the discretion of the operator. Both operator and patient were aware of the assigned treatment.
The procedure data and adjunctive medication were described previously11. All patients were pretreated with aspirin and clopidogrel, and heparin was administered to maintain the activated clotting time > 250 seconds. Glycoprotein receptor antagonists were used at the discretion of the operator. Clopidogrel was continued for one year after stent implantation and aspirin indefinitely. Clopidogrel treatment was extended to 12 months after the last stent implantation both in patients who had a re-PCI performed due to clinical restenosis, and in patients who were treated with PCI for a non-target lesion in the follow-up period.
Follow-up
Re-angiography was performed in connection with recurrent symptoms and in any case at six months, and all patients were followed clinically for 15 months after stent implantation with continuous monitoring of the angina status. A non-scheduled angiogram performed 3-6 months after stent implantation replaced that at six months.
Study endpoints, definitions and data analysis
The primary endpoint of the SCANDSTENT study was the angiographic reduction in the minimal lumen diameter of the target lesion11. This study focuses upon major adverse cardiac events (MACE) occurring within 15 months after stent implantation: death, myocardial infarction and target vessel revascularisation (TVR) in addition to the frequency of stent thrombosis. Death was regarded as cardiac in the absence of any other clear non-cardiac cause. A Q-wave myocardial infarction was defined as the development of new Q-waves lasting > 0.4 second in two or more contiguous leads in connection with chest pain and/or a rise in CK-MB. A non-Q-wave myocardial infarction was defined as a total creatine kinase elevation > 2 times the upper normal limit with a concomitant increase in creatine kinase MB blood concentration in the absence of pathologic Q-waves. TVR was defined as repeat revascularisation of a target vessel including side branches of bifurcations in the presence of myocardial ischaemia and a significant stenosis in the vessel. Thus, all cases of TVR should be clinically driven.
Stent thrombosis was defined as definite in case of angiographically visible signs of a contrast filling defect in the target lesion in connection with an acute coronary syndrome or pathologic confirmation of stent thrombosis. A probable stent thrombosis was defined as any unexplained death within 30 days or a target vessel myocardial infarction (without angiographic documentation), and a possible stent thrombosis was present in case of any unexplained death later than 30 days after stent implantation15.
It was calculated that a total of approximately 300 patients should be included in order to detect a 40% relative reduction in MACE rate at one year (power 0.8, type 1 error 0.05)1. Differences in categorical variables were analysed by the Chi-square test or by Fisher’s exact test. Continuous variables were analysed using the Student’s t-test for unpaired samples. The Kaplan Meier method was used to create survival estimates, and the log-rank test was used to test differences in these estimates. All p-values were 2-sided.
Results
During a 20 month period, 322 patients were enrolled in the trial at four Danish PCI centres. The baseline demographic characteristics of the two patient groups were well matched (Table 1).
Figure 1 shows the distribution of lesions as classified by their primary and additional lesion type showing that 72 patients had an index lesion with more than one complexity (e.g. an occlusion could also be angulated and/ or a bifurcation).
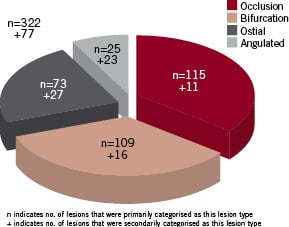
Figure 1. Lesion types with additional complexities.
Of 142 patients with multivessel disease, 112 (35%) had PCI performed in at least one other vessel in connection with treatment of the index lesion. The mean length (standard deviation [SD]) of the index lesion was 18.8 (13.0) mm in the SES group vs 17.2 (11.1) mm in the BMS group (non significant [NS]).
Angiographic findings
Follow-up coronary angiography was available in 91% of the patients. At follow-up, the minimal lumen diameter was larger, the diameter stenosis less severe, and the binary restenosis rate considerably reduced after treatment with SES compared with BMS11.
Clinical outcomes
The clinical outcomes of the patients are listed in Table 2.
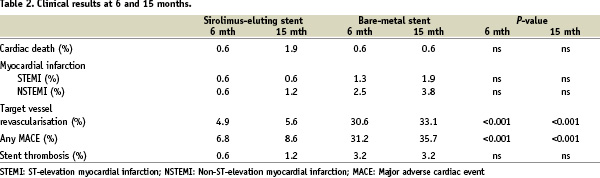
Within 15 months three patients in the SES and one patient in the BMS group died, while three patients in the SES and nine patients in the BMS group suffered a myocardial infarction (both NS). TVR was performed in nine patients (5.6%) in the SES vs 52 patients (33.1%) in the BMS group (P<0.001). In addition, 32 patients (10%), 14 in the SES and 18 in the BMS group, developed one or more significant non-target lesion that was treated during the follow-up period.
The rate of MACE was 8.6% in the SES group vs 35.7% in the BMS group (P<0.001). From six to 15 months after stent implantation three events occurred in the SES group vs seven events in the BMS group (P=0.36). Kaplan-Meier estimates of the event-free survival are delineated in Figure 2 showing that the initial clinical benefit obtained with the SES was maintained during the following observation period.
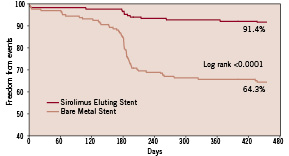
Figure 2. Kaplan-Meier estimates of survival, free from MACE.
A considerable increase in the rate of MACE occurred in the BMS group around the period of the six month follow-up angiography, mainly due to an accumulated rate of TVR. This phenomenon is to be considered a consequence of that re-examinations of patients with recurrent angina in the intervening period were postponed until the already scheduled angiography rather than the “oculostenotic” reflex. Indeed, our clinical events committee assured that all TVR performed were clinically driven.
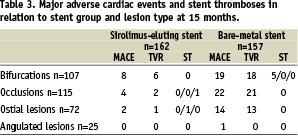
We did not observe any increase in late development of clinical restenosis (“catch-up” effect) in the SES group. MACE occurred in all types of lesions except in the angulated lesions in the SES group.
Stent thromboses
Although clopidogrel treatment was prolonged in patients in whom either TVR or PCI for a de novo non-target lesion was performed during the follow-up period, 80% of the patients (90% in the SES group, 70% in the BMS group) had stopped dual antiplatelet therapy and took aspirin only at 15 months after the index treatment.
There were seven cases of stent thrombosis during the 15 month follow-up period (five in the BMS and two in the DES group) of which five were categorised as definite, one as probable and one as possible. Four cases occurred within one month, two occurred lately (between 30 days and one year) and one very lately (after one year). The five definite cases of stent thrombosis all occurred in BMS bifurcations within six months (Table 3).
All patients who had an early or a late stent thrombosis were on clopidogrel at the time of stent thrombosis except one who developed allergy to clopidogrel early after stent implantation and received ticlopidine for three months only. Of the 162 patients who were alive in the SES group, 143 stopped their clopidogrel treatment 12 months after the index treatment. One of these patients (0.7%) died suddenly after 438 days (73 days after having stopped taking clopidogrel) and was thus categorised as having a possible SES thrombosis very lately.
Discussion
The major finding of this study is the clinical benefit of implanting drug eluting stents in patients with complex coronary artery lesions through 15 months, at which time the majority of patients had reduced their antiplatelet medication to aspirin only. The rate of death and myocardial infarctions was similar in the two groups, whereas implantation of SES markedly reduced the frequency of TVR and MACE compared with BMS in patients with complex coronary artery lesions. Only one (possible) case of (very) late SES thrombosis was observed. Long-term follow-up of SES implantation in simple lesions have shown a tendency towards convergence of the MACE curves after six months16. Our results differ from those of the RAVEL trial in that the MACE curves of the two groups of the present patients with complex coronary lesions seem to continue their divergence after six months.
The MACE rate in the BMS group of the present study is of the same magnitude as that of another study evaluating the effect of paclitaxel stent implantation in patients with somewhat complex lesions6. Thus, this relatively high MACE rate reflects the complexity of the course of these patients when treated conventionally and according to the recommendations10.
The MACE rate in our SES group is comparable with that seen in patients having SES implanted in simple lesions, indicating that our patients receive a treatment that is not below the usual standard of high volume PCI centres. In general, several studies indicate that patients who have SES implanted seem to fare somewhat better than those with paclitaxel stents17.
Recent studies have shown short-term benefit of implanting drug eluting stents in patients suffering an acute myocardial infarction18-20, in small vessels21, in long lesions2,6 and in diabetic patients in general22. Long-term outcome of these studies will contribute to our experience with drug-eluting stent implantation in complex coronary lesions.
Only a few of the previous studies have focused on patients with such complex lesions as bifurcations, total occlusions, ostial and angulated lesions8,23. In addition, long-term experience after implantation of drug eluting stents is scarce, and randomised trials have limited the inclusion of patients to those with relatively simple coronary lesions16,24. Recently published large registries of all-comer patients have indicated that late stent thrombosis may occur more frequently in drug eluting stents compared with BMS25,26.
That a considerable number of our patients were treated for additional lesions in the coronary tree either simultaneously with the treatment of the index lesion or later, underlines that a considerable fraction of this group of patients with complex coronary artery disease indeed suffer an aggressive atherosclerotic disease. The mandatory medical treatment including both antithrombotic and lipid lowering therapy is probably as important as the re-vasculatory procedures in these patients with regard to long-term outcome.
Our findings are consistent with recent follow-up reports of patients treated with SES for relatively simple coronary lesions16,24. Together with those results, our data suggest that the fear of finding a late “catch-up” or delayed restenosis effect after SES implantation, a phenomenon known from vascular brachytherapy27, has not come to fruition within a nine month period following the mandatory six month control angiography.
In the present study, MACE occurred in all lesion types in patients who had BMS implanted in their complex lesion, whereas 88% of MACE in the SES group occurred in patients with bifurcation lesions, indicating that drug eluting stents may virtually eliminate the clinical restenosis problem in a variety of patients with non-bifurcation complex lesions. Still, the reduction in MACE through 15 months in the SES compared with the BMS group was substantial in patients with all lesion types, even in those patients who had lesions located in bifurcations. In addition, the tendency to a higher rate of stent thrombosis in bifurcation lesions after BMS implantation calls for precaution using BMS in bifurcations, provided these findings can be confirmed in larger scale studies. In the present study approximately 50% of the patients with bifurcation lesions had a stent implanted in the side branch11. A recent study indicated that side branch stenting with SES does not result in any clinical benefit28. However, bifurcations remain a true complex entity, the treatment of which needs to be optimised29,30.
Implantation of drug-eluting stents may induce pathologic changes in the walls of the coronary vessels31, and together with others we have previously stressed the importance of long-term evaluation of the clinical outcome after implantation of drug eluting stents in coronary arteries11,32. Recent reports have raised concerns about the use of drug eluting stents due to a risk of late stent thrombosis and myocardial infarction, especially in patients with complex disease or complex coronary artery lesions12,14,33. Accordingly, the American Food and Drug Administration has recently issued the recommendation that drug eluting stents be used with caution in patients whose coronary artery lesions differ from those in patients included in the pivotal trials (i.e. “off-label”).
Our patients all had complex coronary lesions and thus their clinical outcome contributes to the long-term experience with drug-eluting stents in patients with “off-label” lesions. We found neither any increased mortality nor any excess rate of myocardial infarction by using SES in these complex coronary lesions. It should be stressed that the number of patients in the present study was limited and the trial was not powered to draw conclusions about the long-term safety of drug eluting stents. Still, our findings contribute to a recent meta-analysis based on individual data of nearly 5,000 patients randomised to receive a SES or a BMS in their coronary artery lesion, in which no significant differences were observed in all-cause mortality or non-fatal myocardial infarction between the groups34.
Conclusions
SES implantation improves the clinical outcome compared with BMS in patients with a variety of complex lesions without any increase in delayed restenosis or excess rate of adverse events out to 15 months after stent implantation and after discontinuation of clopidogrel therapy. Still, longer-term follow-up of these patients is mandatory.

