Abstract
Aims: We sought to determine the efficacy of enoxaparin in unselected patients with STEMI treated with primary percutaneous coronary intervention in clinical practice.
Methods and results: In a retrospective analysis of the prospective MITRA-plus registry we compared the outcomes of patients with primary PCI and either enoxaparin or unfractionated heparin. A total of 2,655 patients with STEMI < 12 hours were included in this analysis, 374 (14%) were treated with enoxaparin and 2,281 (86%) with unfractionated heparin. In the univariate analysis enoxaparin reduced mortality (1.6% versus 6.0%, < 0.001), fewer non-fatal reinfarctions (1.9% versus 3.8%, p=0.05) and no significant difference in major bleeding (5.6% versus 7.2%, p=0.2) was observed. In the multivariable propensity score analysis enoxaparin was associated with a reduction in the combined endpoint of death and non-fatal reinfarction (odds ratio 0.42; 95% CI 0.2-0.8). This advantage was observed both in subgroups without (odds ratio 0.33 95% CI 0.1-0.8) and with GP IIb/IIIa inhibitors (odds ratio 0.44, 95% CI 0.2-1.0).
Conclusions: Our data suggest that in unselected patients with STEMI treated with primary PCI enoxaparin compared to unfractionated heparin reduces the combined endpoint of in-hospital death and reinfarction and does not increase severe bleeding complications.
Introduction
In patients with STEMI, primary percutaneous coronary intervention for ST segment elevation myocardial infarction therapy with aspirin, thienopyridines and unfractionated heparin (UFH) is the standard of care and has been shown to reduce the rate of death or non-fatal myocardial infarction in randomised clinical trials. Therefore these agents are recommended in current STEMI guidelines1,2. UFH has some shortcomings, including its indirect mechanism of thrombin inhibition, direct platelet activation, the inability to inactivate clot-bound thrombin, the tendency to promote thrombin binding to fibrin, and avid and non-specific protein binding3. The low molecular weight heparin enoxaparin is a potential replacement for UFH. It provides a more stable level of anticoagulation without the need for therapeutic monitoring. Furthermore, it demonstrates less protein binding and platelet activation and relatively greater inhibition of the coagulation cascade compared to UFH because it has a a ratio of 4.3:1 in its anti-factor Xa to anti-factor IIa activity4. In randomised clinical trials, the low-molecular-weight heparin enoxaparin has decreased ischaemic complication rates in patients with STEMI who are treated with fibrinolysis5-11. Little is known about the comparative efficacy and safety of enoxaparin in primary PCI. In patients with elective PCI, enoxaparin was as effective as UFH, but associated with less bleeding complications12. In addition, in patients with STEMI initially treated with fibrinolysis undergoing PCI, during follow-up enoxaparin reduced the combined endpoint of death and reinfarction until day 30 in the ExTRACT-TIMI 25 trial13. The aim of our study was to determine the use and value of enoxaparin in unselected patients with primary PCI in the German MITRA-Plus registry.
Methods
The MITRA PLUS registry
The Maximal Individual Therapy of Acute Myocardial Infarction PLUS registry (MITRA PLUS) is a German prospective, multicentre, observational data pool of current treatment of acute coronary syndromes14. Since 1994 more than 45,000 consecutive patients in 390 hospitals were included in the MITRA PLUS registry. The MITRA PLUS registry consists of consecutive subregistries that have been earlier described in detail: the 60 minutes Myocardial Infarction Project (60 minutes MIP), Maximal Individual Therapy in Acute Myocardial Infarction (MITRA), Myocardial Infarction Registry (MIR) Acute Coronary Syndromes registry (ACOS) and GOAL.
Definitions
STEMI was diagnosed in the presence of the two following criteria: persistent angina pectoris for >20 minutes and ST-segment elevation of > 1 mm in > 2 standard leads or > 2 mm in > 2 contiguous precordial leads, or the presence of a left bundle branch block. It was later confirmed by the elevation of cardiac enzymes to more than twice the upper limit of normal.
Major bleeding was defined as any intracranial bleeding, bleeding associated with the need for blood transfusion or any other clinically relevant bleeding leading to a medical or surgical intervention. Reinfarction was defined as recurrent angina and recurrent increase of CK-MB over 50% of the last level or over the norm (if CK-MB has already normalised) or angiographic documentation of re-occlusion.
Data collection
The patients were recruited from hospitals in Germany, including community hospitals as well as tertiary centres. On admission, data on patient characteristics including age, gender, cardiovascular risk factors, concomitant diseases, prior myocardial infarction, prior stroke, prior cardiovascular interventions and chronic medical treatment, as well as data on symptoms and pre-hospital delay were recorded. During the hospital stay data on electrocardiographic findings, biochemical markers, reperfusion treatment, pharmacological treatment and interventional procedures were documented. At discharge, outcome and major cardiovascular and cerebrovascular adverse events were recorded as well as risk assessment and medication at discharge.
Data sampling, control of the data quality, generation of queries, and statistical handling of the data were performed centrally in the Institut für Herzinfarktforschung in Ludwigshafen aan der Universität in Heidelberg, Germany.
Patients
For this retrospective analysis, we created a subgroup of STEMI patients treated with primary PCI and UFH or enoxaparin within 24 hours after admission. Patients treated with both UFH and enoxaparin and patients receiving low molecular weight heparins other than enoxaparin were excluded from the analysis.
Statistics
The absolute numbers, percentages, medians as well as 25% and 75% quartiles were used for the description of the patient population. For categorical variables we used the Chi-square or Fisher’s exact test and calculated the odds ratios with 95% confidence intervals. A p-value of < 0.05 was considered significant. A multivariable propensity score analysis was performed adjusting for age, gender, prior myocardial infarction, diabetes mellitus, prior stroke, peripheral arterial disease, smoking habit, hyperlipidaemia, renal insufficiency, pre-hospital delay and cardiogenic shock. The analyses were performed with the SAS statistic package (SAS Institute version 8.2, Inc, Cary, NC, USA).
Results
Between 2000 and 2004 a total of 2,655 patients fulfilled our inclusion criteria for this analysis, 374 (14%) were treated with enoxaparin and 2,281 (86%) with UFH. The baseline characteristics were comparable between the two groups (Table 1). The only significant difference was the higher rate of cardiogenic shock in the UFH group.
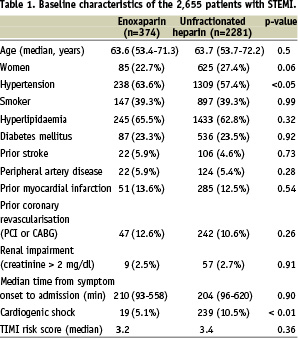
The acute therapies applied within the first 24 hours after admission are shown in Table 2. While clopidogrel was given more often in the enoxaparin group, GP IIb/IIIa inhibitors were used more often in the patients with UFH. The rates of use of abciximab (38.2% v. 42.6%) and tirofiban (32.9% vs. 40.0%) did not differ significantly, while eptifibatide was used more often with enoxaparin (32.4% vs. 19.4%).
All patients were prospectively followed until hospital discharge. The mean duration of hospital stay was 10 (7-13) days in both groups. In the total patient population, enoxaparin was associated with a significant lower incidence of death and a trend towards less reinfarction, while there was no significant difference in stroke or major bleeding complications (Figure 1). The incidence of bleeding complications with the need for blood transfusions was 0.6% and 1.8% with enoxaparin and UFH, respectively. We observed a higher rate of death (5.5%) the combined endpoint (9.0% vs. 6.8%) in patients with major bleeding compared to those without bleeding.
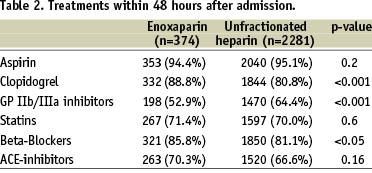
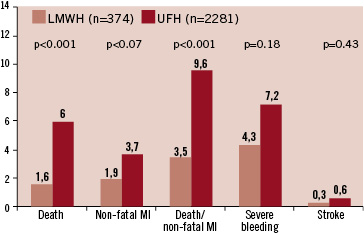
Figure 1. In-hospital clinical events in the patients with STEMI treated with enoxaparin or unfractionated heparin.
We divided patients into two subgroups: patients treated with and without GP IIb/IIIa inhibitors. As shown in Figure 2, the combined endpoint of death and non-fatal myocardial infarction was reduced by enoxaparin in both subgroups. In a multivariable propensity score analysis for the occurrence of death and non-fatal myocardial infarction until discharge, the use of enoxaparin was an independent predictor of a better clinical outcome in the total group and both subgroups (Table 3).
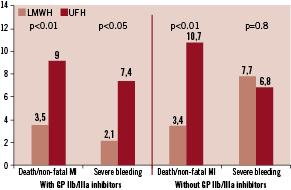
Figure 2. In-hospital clinical events in the subgroup of patients with and without GP IIb/IIIa inhibitors.
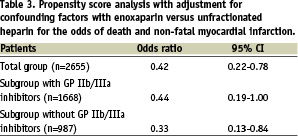
In two other important subgroups, enoxaparin again was associated with a reduction in the combined endpoint death and non-fatal reinfarction: patients > 75 years (n=455) 8.8% versus 14.3% and patient with impaired renal function (n=65) 12.5% vs. 45.6%.
The benefit of enoxaparin was maintained during the 12-month follow-up after discharge. The mortality after discharge was 2.8% with enoxaparin and 4.2% with UFH. The cumulative incidence of the combined endpoint of death and non-fatal reinfarction after admission until 12 months was 9.7% vs 17.0% after enoxaparin and UFH, respectively.
Discussion
In our retrospective study we observed a reduction in the combined endpoint of total mortality and myocardial reinfarction with enoxaparin compared to UFH in unselected patients with STEMI treated with primary PCI in clinical practice. Recently, the results of the large clinical multicentre, double-blind, randomised EXTRACT-TIMI 25 trial with over 20,000 patients with STEMI treated with fibrinolysis were published10,11. It compared enoxaparin initiated with an intravenous bolus of 30 mg followed by 1 mg/kg twice daily s.c. for up to eight days and unfractionated heparin initiated with a bolus of 60 IU/kg followed by an infusion of 12 IU/kg/h for 48 hours. The primary endpoint, a composite of death and reinfarction at 30 days, was significantly reduced with enoxaparin (10% vs 12%, p<0.001). While there was no increase in intracerebral bleeding, there were significantly more major bleeding with enoxaparin. In addition, in patients undergoing PCI during the hospital course, enoxaparin was associated with a reduction of ischaemic events.
So far there is only little data available about the use of enoxaparin in patients with primary PCI. In a small substudy of the WEST trial, anti-Xa activity was measured 55 minutes after a subcutaneous administration of 1 mg/kg of enoxaparin in patients with primary PCI for STEMI. Of these patients, 87% had anti-Xa levels below 0.5. An additional i.v. bolus of 0.3-0.5 mg/kg at the time of PCI achieved in all patients a therapeutic anti-Xa level of 0.8-1.0816. In the ADVANCE-MI trial, patients scheduled for primary PCI were randomised to receive half-dose tenecteplase and eptifibatide, or placebo and eptifibatide and in a second randomisation UFH or enoxaparin (open label bolus of 0.4 mg/kg not to exceed 40 mg). This study was stopped prematurely for low enrolment after 148 patients. In this small study, there was no difference between UFH and enoxaparin with respect to ischaemic events or bleeding complications17. Therefore, our analysis is the one of the first to determine the effectiveness of enoxaparin in primary PCI. Enoxaparin reduced both death and myocardial infarction and showed a trend towards fewer bleeding complications. The groups were well balanced, except for the incidence of cardiogenic shock. This explains the large difference in mortality between the two groups in the univariate analysis. Therefore we performed a propensity score analysis, including cardiogenic shock, to adjust the results for this important and clinically relevant difference. However, even after adjustment, the advantage of enoxaparin remained significant.
A difference occurred in concomitant antiplatelet medication with a higher use of clopidogrel in the enoxaparin group, and a higher use of GP IIb/IIIa inhibitors in the UFH group. This might be due to the fact that in Germany the combined use of enoxaparin and GP IIb/IIIa inhibitors is not a standard regimen, despite the positive results in randomised trials18. However, it is unlikely that the differences in concomitant medication account for the differences in clinical event rates in the two groups since the more potent platelet GP IIb/IIIa inhibitors were used more often in the UFH. When we divided the patients into subgroups treated with or without GP IIb/IIIa inhibitors, we observed significant reductions in the combined endpoint in both subgroups, showing a consistent benefit of enoxaparin.
The advantages and disadvantages of UFH and low molecular weight heparins have been discussed extensively. The ease of administration and the lack of the need for monitoring make enoxaparin a convenient alternative to unfractionated heparin in patients with STEMI, especially in clinical routine.
One explanation for our observations might be that UFH is closely and well monitored in clinical trials, and thus is associated with a more stable level of anticoagulation than what might be achieved in clinical practice. On the other hand, the efficacy and safety of enoxaparin is not linked to close monitoring, except for an adjustment of the dose in patients with impaired renal function. Therefore this advantage seems to be even more important in clinical practice.
Here the assumed more stable level of anticoagulation achieved by enoxaparin was associated with a reduction of ischaemic complications. We did not observe a significant difference in severe bleeding complications between the two groups, but numerically less bleeding with enoxaparin, especially in the subgroup treated with GP IIb/IIIa inhibitors. This is in line with the findings of the STEEPLE trial performed in patients with elective PCI12. Again, the high inter- and intra-individual variability of heparin without close monitoring might contribute to these results. The results of the recently presented HORIZONS study underlines the importance of avoiding bleeding complications by a maintained anti-ischaemic efficacy with an effective antithrombin, in this case bivalirudin.
Limitations
Our analysis was derived from a registry and not a randomised clinical trial. However, there were no major differences between the groups in the baseline characteristics and concomitant medications. Still, a selection bias can not be fully excluded, even after adjusting for multiple predictors of outcome. We do not have information about the duration of treatment with either enoxaparin or unfractionated heparin and the aPTTs achieved with unfractionated heparin, all factors which might have influenced the clinical event rate. However, potential differences in the duration of treatment reflect actual clinical practice, possibly favouring a longer treatment with the more convenient subcutaneous regimen with enoxaparin, without the need for laboratory controls.
Conclusions
Our data suggests that in unselected patients with STEMI treated primary PCI, enoxaparin compared to unfractionated heparin reduces the combined endpoint of in-hospital death and reinfarction and does not increase severe bleeding complications.
Therefore a randomised trial seems warranted to define the role of enoxaparin in primary PCI.
Acknowledgements
This study was supported by MSD Germany and Sanofi-Aventis Germany.

