Abstract
Aims: The efficacy of paclitaxel-eluting stents (PES) in patients with ST-segment elevation acute myocardial infarction (STEMI) has not been demonstrated yet. The aim of the present study was to evaluate the efficacy and safety of PES in patients with STEMI.
Methods and results: A meta-analysis from three randomised trials that compared PES and bare-metal stents in patients with STEMI was performed. Overall, 925 patients were included: 459 allocated to PES, and 466 to bare-metal stents (BMS). The rates of major adverse events (i.e. death, reinfarction, and target vessel revascularisation at 6-12 month follow-up) was compared for patients with PES and BMS. Compared to patients with BMS, a significant reduction in the incidence of events (9.1% vs. 13.9%, p=0.02; OR 0.62; 95%, CI: 0.41-0.93), and target vessel revascularisation (4.7% vs. 8.3%, p=0.03; OR 0.54; 95%, CI 0.31-0.94) was found in patients with PES. The rates of death and reinfarction were similar in BMS and DES patients.
Conclusions: The use of PES in patients with STEMI is associated with a significant reduction in MACE and need for new revascularisations.
Introduction
Several randomised trials have shown that, compared to bare metal stents (BMS), drug-eluting stents (DES) reduce binary angiographic restenosis and the need for repeat revascularisation1. Given the propensity of infarct-related arteries to stent thrombosis ST-segment elevation myocardial infarction (STEMI) was initially considered a contra-indication for DES implantation2. Moreover, the clinical impact of angiographic restenosis is probably lower in infarcted areas of myocardium. The use of DES in primary percutaneous coronary intervention (PCI) for STEMI has therefore been less frequent than in other clinical settings. Recently, several randomised trials have demonstrated that the sirolimus-eluting stent (SES) (Cordis, USA) reduces not only binary angiographic restenosis, but also the rate of new revascularisation procedures compared to BMS3-5. In contrast, the recently published PASSION trial failed to demonstrate a clinical benefit of the paclitaxel-eluting stent (PES) (Boston Scientific, USA) in this clinical setting, with no significant difference in repeat revascularisation rates between the PES and BMS patient groups. However, the PASSION study may have been underpowered to show the benefit of PES in STEMI6. To overcome this limitation, we performed a meta-analysis of three randomised trials which compared PES with BMS in patients with STEMI.
Methods
Selection of the trials
We conducted a computerised bibliographic search of the MEDLINE database (National Library of Medicine, Bethesda, MD, USA) and in the abstract supplements of five major scientific meetings – the European Society of Cardiology, the American College of Cardiology, American Heart Association, EuroPCR, Transcatheter Cardiovascular Therapeutics – through April 2007. We selected randomised trials which compared PES and BMS in patients with STEMI. We found three trials: PASSION (the Paclitaxel-Eluting Stent versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation), BASKET (BAsel Stent Kosten Effektivitäts Trial)-MI, and HAAMU-STENT (Helsinki Area Acute Myocardial infarction treatment re-evalUation – Should The patient get a drug-Eluting or a Normal sTent)6-8. In the PASSION trial, 619 patients with STEMI within 12 hours after symptoms onset underwent primary PCI and were randomly allocated to PES (n=310) or BMS (n=309). In the BASKET-MI study, 217 patients with STEMI undergoing primary PCI were allocated in a 1:1:1 design to BMS (n=75), SES (n=76) or PES (n=67). The 142 patients allocated to the BMS and PES arms were included for this analysis. Finally, the HAAMU study allocated 213 patients with STEMI within 12 hours after symptoms onset to thrombolysis or abciximab facilitated PCI randomised to BMS (n=82) or PES (n=82) (HAAMU-STENT trial). Our study population therefore consists of 925 patients: 459 allocated to PES and 466 to BMS.
Statistical analysis
The review was conducted according to the Quality of Reports of Meta-Analyses of Randomised Clinical Trials (QUOROM) recommendations. The Reviewer Manager (Rev Man) version 4.2 for Windows (The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen, Denmark), and the SPSS 10.0 (Chicago, Illinois, USA) statistical packages were used.
Quantitative variables are expressed as mean values, and discrete variables as percentages. The odds ratio (OR) for death and reinfarction, and their 95% confidence intervals (CI) were used as parameters of safety, and the OR for target vessel revascularisation (TVR) and major adverse cardiac events (MACE) were used as parameters of efficacy of PES in comparison with BMS. MACE was defined as the combination of death, re-infarction, and target vessel revascularisation in PASSION, and HAAUMU-STENT trials, whereas in the BASKET-MI trial, MACE was defined as cardiac death, re-infarction, or target vessel revascularisation. CI were calculated comparing PES with BMS rates using data for each study and for the pooled population (intention-to-treat basis). The combined effect for the heterogeneity was calculated by taking the inverse variance estimated. The effect of each study was weighted for its number of patients. Usually, the Der Simonian and Laird random effects and the fixed effect models are used when p value for statistics Q test of heterogeneity is <0.05, and >0.05, respectively. However, the test for heterogeneity (Q) is likely to be underpowered in our study given the small number of trials included. Because of that, in case Q-test for heterogeneity showed p>0.05, we provided the results of both fixed effect and random effect models. Associations were considered statistically significant when p<0.05.
Results
Characteristics of the trials included
The characteristics of the trials included are shown in Table 1.
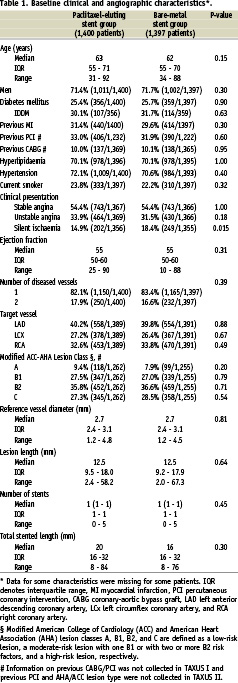
The PASSION trial was performed in two centres, whereas both the BASKET-MI and the HAAMU-STENT studies were single-centre randomised studies6-8. Routine angiographic follow-up was performed only in the HAAMU-STENT study. Due to the initial study design (comparison of thrombolysis and abciximab prior to coronary angiography), 45% of patients included in the HAAMU-STENT study had received thrombolytic therapy, whereas all patients in the remaining two studies underwent PCI as the initial reperfusion strategy.
EFFICACY OF PES VERSUS BMS
Clinical follow-up was obtained in 914 patients (98.5% of the patients). No significant reduction of TVR or MACE was noted in the individual trials between patients with PES compared to those with BMS. In the pooled analysis, there was no heterogeneity among the trials, both for incidence of MACE (Q-test for heterogeneity: p=0.42) and TVR (Q-test for heterogeneity: p=0.50).
Overall, the incidence of MACE was significantly lower in patients allocated to PES in comparison with patients allocated to BMS (9.1% vs. 13.9%, p=0.02; OR 0.62; 95% CI: 0.41-0.93) as shown in Figure 1A.
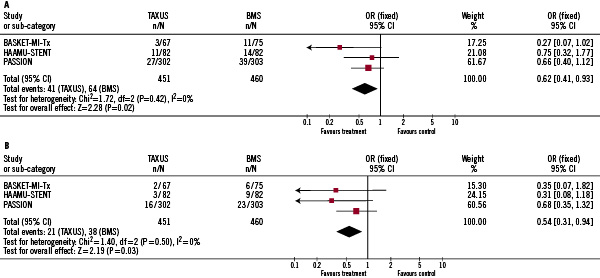
Figure 1. Efficacy of PES (paclitaxel eluting stent) compared to BMS (bare metal stent) in patients with STEMI (ST segment elevation myocardial infarction). Fig. A. Comparison between Taxus and BMS in terms of incidence of MACE during follow-up. Fig. B. Comparison between Taxus and BMS in terms of incidence of TVR during follow-up.
The rate of TVR was also significantly lower in patients with PES (4.7% vs. 8.3%, p=0.03; OR 0.54; 95% CI 0.31-0.94), seen in Figure 1B. We noted a 4.8% absolute risk reduction in the rate of MACE and a 3.5% reduction for the need of TVR (relative risk reduction 35%, and 43%, respectively). Compared to BMS, the number needed to treat to prevent one MACE and one TVR by implantation of PES in patients with STEMI was 21, and 28, respectively. When a random effects model, instead of fixed effects model, was used, results were similar (OR for MACE, and TVR 0.54 [95% CI: 0.31, 0.94; P=0.03], and 0.55 [95% CI: 0.32, 0.96; P=0.04], respectively).
Safety of PES in the setting of PCI for STEMI
PASSION was the only trial which analysed the incidence of stent thrombosis. Similar rates were found between patients allocated to PES or BMS (1% in both groups)6.
In the pooled analysis, no significant differences were found in the incidence of death and reinfarction. We found no heterogeneity among the trials (Q-test for heterogeneity: p=0.18, and p=0.17 for death and reinfarction, respectively). Mortality rate during follow-up was 5.1%, and 6.1% for patients allocated to PES, and BMS, respectively (p=0.50; OR 0.82; 95% CI 0.47-1.45), Figure 2A.
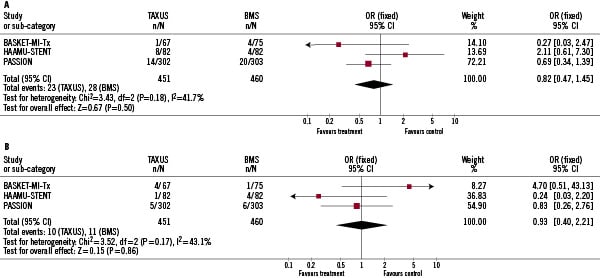
Figure 2. Safety of PES (paclitaxel eluting stent) vs. BMS (bare metal stent) in patients with STEMI (ST segment elevation myocardial infarction). Fig. A. Comparison between PES and BMS in terms of mortality during follow-up. Fig. B. Comparison between PES and BMS in terms of incidence of reinfarction during follow-up.
The incidence of reinfarction was also similar in patients allocated to PES, and BMS (2.2% vs. 2.4%; p=0.88; OR 0.93; 95% CI: 0.40-2.21), Figure 2B. When the random effects model, instead of a fixed effects model was used, results were similar (OR for death, and re-infarction 0.86 [95% CI: 0.34, 2.28; P=0.76], and 0.94 [95% CI: 0.24, 3.80; P=0.94], respectively).
Discussion
In this meta-analysis of three randomised trials comparing PES and BMS in patients with STEMI, a 4.8% absolute risk reduction in the rate of MACE and 3.6% in the need for TVR was noted (relative risk reduction 35%, and 43%, respectively). Compared to BMS, the number needed to treat to prevent one MACE and one TVR by implantation of PES in patients with STEMI was 21, and 28, respectively.
Clinical impact of angiographic restenosis after primary PCI for STEMI
Primary PCI has been accepted as the default reperfusion strategy for STEMI if it can be performed rapidly in an experienced centre9. One of the limitations of primary PCI is that recurrent ischaemia, mainly due to restenosis, occurs in more than 20% of the patients in the following months after STEMI. Moreover, angiographic restenosis after primary PCI for STEMI may hinder recovery of left ventricular ejection fraction during follow-up, especially when an angiographic restenosis with total vessel occlusion occurs10. Finally, in one study, the authors demonstrated that patients with re-occlusion of the infarct-related artery after primary PCI had a higher one-year mortality rate11.
Effectiveness of DES in patients with STEMI
The effectiveness of DES during PCI for STEMI has been studied in randomised trials with a significant reduction of angiographic restenosis and TVR in this setting with the use of SES3,4. The STRATEGY trial, included 175 patients with STEMI. Compared to BMS, treatment with SES was associated with a significant reduction in the incidence of MACE (19 vs. 50%, p<0.01), and TVR (7 vs 20%, p=0.01)3. In the TYPHOON study, SES significantly reduced MACE (7% vs 14%, p<0.01), and TVR (6% vs. 13%, p<0.01) compared to BMS in 700 patients with STEMI4. Similar results were obtained in the SESAMI trial in 320 patients (TVR rate 5 vs. 13% in patients allocated to SES, and BMS, respectively; p<0.05)5.
In contrast, the randomised trials using PES in patients with STEMI have not yielded significant results. In the PASSION study, 619 patients with STEMI were randomly assigned to PES or BMS6. In this study, patients allocated to PES had a lower incidence of MACE, which was, however, not statistically significant (9 vs. 13%, p=0.12). Moreover, the incidence or TVR was not significantly reduced in patients with PES (5 vs. 8%, p=0.23)6. The absence of significant clinical benefit of PES in the PASSION trial may have several potential explanations. First, the anti-proliferative effect of the PES is lower with higher mean values of in-stent late loss compared to SES. Since in-stent late loss after DES implantation is associated with a reduction in the rate of TVR, this effect is of clinical and prognostic significance1. Second, in the PASSION study, no routine angiographic follow-up was performed. It is known that routine angiographic follow-up may increase rates of TVR both in patients treated with BMS and DES. Third, the study population included in the PASSION trial was at relatively lower risk of restenosis and TVR than, for example, patients in the TYPHOON trial. In the PASSION trial, mean reference vessel diameter, mean stented length, and the proportion of patients with diabetes was 3.2 mm, 19 mm, and 11% compared to 2.8 mm, 21 mm, and 16%, respectively, in the TYPHOON trial. DES reduce repeat revascularisation rates mostly in patients with a high risk of clinical restenosis12. We recently performed a meta-regression analysis from 31 randomised trials (12,060 patients) that compared DES and BMS, in order to evaluate the influence of the baseline risk of clinical restenosis and the clinical benefits derived from the use of DES13. In that study, the risk profile of each study was considered to be the rate of revascularisation in patients allocated to BMS in each trial, and the clinical benefit of using DES was defined as the NNT for TLR in each of the trials. After weighting by the number of patients included in each trial, we found a significant relationship between both variables: number needed to treat: 31.1–1.2 (TVR in BMS patients) (95% CI of β parameter: –1.7, –0.6; p<0.001). Therefore, with an increased risk of clinical restenosis, there is a parallel increase of clinical benefit with the use of DES (Figure 3).
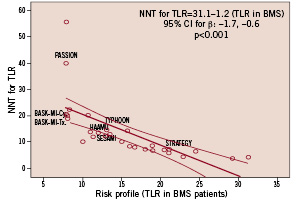
Figure 3. Relationship between the risk of clinical restenosis (incidence of new revascularisation procedures in patients treated with BMS –bare metal stents) and the clinical benefit derived from the use of DES (drug eluting stents) instead of BMS. A significant relationship between both parameters is shown. Most trials involving patients with STEMI (ST segment elevation myocardial infarction) included patients with low risk for clinical restenosis (left side of the figure).
Most of the trials on DES in STEMI included patients at low risk for restenosis and all trials except STRATEGY, had a relatively low incidence of new revascularisations (Figure 3). This supports the hypothesis that, compared to patients without necrotic myocardium in the areas supplied by the treated vessel, angiographic restenosis in the setting of STEMI has less clinical implications. Only the STRATEGY trial showed a >20% rate of new revascularisations in the BMS arm, and –accordingly– the clinical benefit of using DES was stronger with a low number needed to treat for new revascularisations. On the contrary, the PASSION trial showed a very low incidence of clinical restenosis in patients allocated to BMS with a very high number needed to treat for new revascularisations (Figure 3).
In the present study, no significant differences in rates of TVR were found in each separate study. However, there was a significant relative and absolute risk reduction of 43% and 3.5%, respectively, in the need for TVR in the pooled analysis. These data suggest that PES are effective in patients with STEMI in terms of reducing the need for TVR during follow-up. However, compared to other clinical settings, the clinical benefit of PES in patients with STEMI seems to be less important. Absolute risk reduction for the need for TVR was 10%, and 10.3% in TAXUS-IV, and TAXUS-VI trials, respectively, with a number needed to treat for TVR of 1012,14. Conversely, the angiographic benefit seems to be comparable with patients without STEMI. In the HAAMU-stent trial, late lumen loss in patients allocated to taxus stent was similar to that obtained in other TAXUS trials8. Similar findings are noted with the SES, whereas absolute risk reduction in the rate of TVR ranges from 12% to 27% in patients without STEMI (e.g. 12% in the study by Pache et al; 13% in SIRIUS; 14% in SES-SMART; 15% in PRISON-II; 17% in E-SIRIUS; 23% in RAVEL; 24% in DIABETES; and 27% in SCANDSTENT), absolute risk reduction in TVR ranged from 6% to 13% in STEMI trials1,5-8,15-22.
Safety of Taxus stent in primary PCI for STEMI
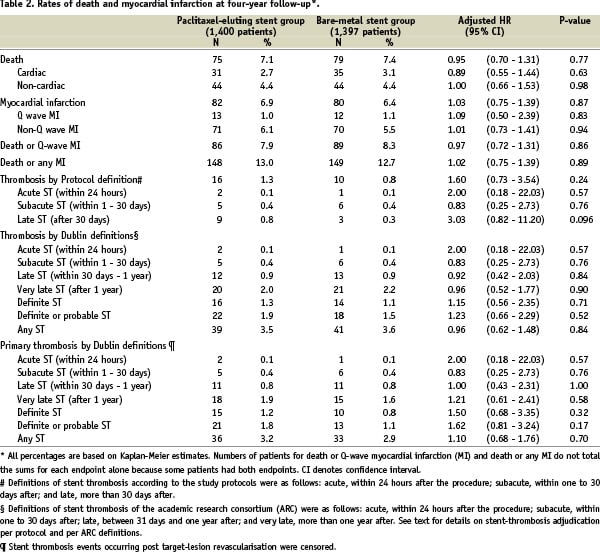
In the PASSION trial, the risk of stent thrombosis within one year after stent implantation was similar in patients treated with PES or BMS (1% in both patient groups)6. The stent thrombosis rate was not reported in the HAAMU-stent and the BASKET-AMI trials. The risk of both death, and reinfarction was similar in patients allocated to PES or BMS in the present pooled analysis from the PASSION, BASKET-AMI, and HAAMU-Stent. Long-term (four to five years) follow-up of randomised trials has documented a slight increase in the risk of stent thrombosis in patients treated with DES more than one year after stent implantation23. Although this seems to have no clinical impact in terms of long-term mortality or risk of myocardial infarction24, longer follow-up of trials using DES in patients with STEMI is mandatory25.
Study limitations
This study has limitations. First, as with other meta-analyses, inclusion criteria may be different among studies. It was not a patient based data meta-analysis, and studies were performed with different designs. Moreover, the total number of patients was relatively small. The efficacy of PES in patients with STEMI will be clarified with the on-going HORIZONS trial comparing PES and BMS in a large population of patients with STEMI.
Conclusions
Although the clinical benefit of DES is probably lower in patients with STEMI than in other clinical scenario, treatment with PES, in comparison with BMS, is associated with a significant reduction in the rate of TVR in these patients. PES are safe, in terms of mortality or re-infarction, at least during the first year after stent implantation. Longer follow-up of these trials, however, is warranted.

