Abstract
Aims: Percutaneous repair of mitral regurgitation (MR) by leaflet apposition using a clip deployed via transseptal catheterisation is undergoing evaluation.
Methods and results: In order to detect the potential for clinically significant left ventricular inflow obstruction after percutaneous repair, we measured mitral valve area (MVA) and mean transmitral gradient (MVG) echocardiographically in 96 patients implanted with a clip followed for up to 24 months.
By planimetry, the mean MVA decreased from 6.0±1.3 cm2 to 3.6±1.2 cm2 (p<0.05) (range 1.9 to 7.6 cm2) after clip placement, and remained unchanged after 24 months of follow-up (3.5±0.8 cm2). The mean MVG increased after clip placement from 1.7±0.9 mmHg to 4.1±2.2 mmHg (p<0.05), and did not increase further to 24 months (3.8±1.9 mmHg). There were no differences in MVA or MVG between patients who received 1-clip (69%) and those receiving 2-clips (31%). Patients with functional MR (23%) had a slightly smaller MVA, both at baseline and after clip placement, but did not differ from degenerative MR patients at later follow-up. After 2 years of follow-up, no patient required surgery for LV inflow obstruction.
Conclusions: Mitral repair with the MitraClip® device for MR decreases MVA without significant mitral obstruction. After 2 years of follow-up, no patient required surgery for LV inflow obstruction, and these results were not influenced by the use of more than 1 clip or the aetiology of MR.
Introduction
Percutaneous mitral valve repair as an alternative to surgical valve repair or replacement for mitral regurgitation (MR) is undergoing evaluation1. One percutaneous method involves leaflet apposition with a clip (MitraClip® device, Evalve, Inc. Menlo Park, CA, USA) deployed via a transseptal catheterisation technique2-5. This approach was first evaluated in a phase I registry and is undergoing evaluation in a randomised, pivotal trial (EVEREST-II) compared to surgery2.
Data from surgical edge-to-edge leaflet repair as originally described by Alfieri and colleagues have demonstrated a reduction in mitral valve area without clinically significant mitral stenosis6-8. In order to detect the potential for clinically significant left ventricular inflow obstruction after percutaneous leaflet apposition, we measured mitral valve area (MVA) and transmitral gradient (MVG) following repair with the MitraClip device. In a preliminary study, we demonstrated a decrease in mitral valve area using the percutaneous technique that was similar to surgery9. In the present study, we are extending these observations to a larger study population, including patients who received 2 clips and those with functional MR, and with longer follow-up.
Methods
Patients were selected for therapy if they met criteria for intervention based on the 1998 and 2006 ACC/AHA Joint Task Force recommendations regarding therapy for valvular heart disease. The population for this analysis included 107 patients with moderate-to-severe or severe MR who were enrolled in the EVEREST phase I registry (n=55) or “roll-in” subjects who were not randomised as part of the EVEREST-II trial (n=52). Baseline characteristics and haemodynamic measurements were analysed and compared for the 96 patients who received clips. All patients were either symptomatic (91%) or if asymptomatic had evidence of left ventricular dysfunction (EF < 60%, LV end-systolic dimension >45 mm for EVEREST-I and >40 mm following release of the 2006 guideline update). Patients with an LV EF < 30% (25% for EVEREST-II) or LV end-systolic dimension >55 mm were excluded. In addition, all echocardiograms were reviewed for key morphologic criteria. Patients were excluded if the baseline MVA was <4.0 cm2, if severe leaflet or annular calcification was present, if the flail width exceeded 15 mm, a flail gap >10 mm, and in patients with a functional aetiology if the coaptation depth was > 11 mm below the annulus or the coaptation length was < 2 mm.
Transthoracic echocardiography was performed at baseline, pre-discharge, and at 12 and 24 months. Baseline and follow-up echocardiograms were analysed by a core echocardiographic laboratory at the University of California at San Francisco, USA4. Mitral regurgitation severity was assessed using an integrative method based on both qualitative and quantitative data as previously described4,10. Continuous wave Doppler of mitral inflow was recorded from the apical four chamber view to assess the peak instantaneous mitral gradient in early diastole and the pressure half-time (PHT) was used to calculate mitral valve area (MVA). Mean mitral valve gradient (MVG) was obtained by planimetry of the spectral Doppler envelope. The MVA was planimetered in the parasternal short axis view at the mitral valve leaflet tips during diastole at the time of maximal opening. Post-clip deployment, the individual planimetered orifice areas were added to derive the total mitral valve area9.
Comparisons of MVA by planimetry and PHT, and MVG were made with paired, matched data at baseline, discharge, and at 12 and 24 months using a general linear model ANOVA with adjusted sum of squares (Minitab statistical software, version 15). Since this model showed a significant difference across follow-up times without any significant interactions between number of clips (1 vs. 2) or aetiology (degenerative vs. functional), paired t-tests were performed to determine where the significance across follow-up times was observed. Unmatched data with different sample sizes available at each time point were also compared with a one-way ANOVA. Insufficient data were available to compare subgroups at 24 months. Results are reported as mean ±standard deviation and comparisons were considered significant if p<0.05.
Results
Baseline characteristics
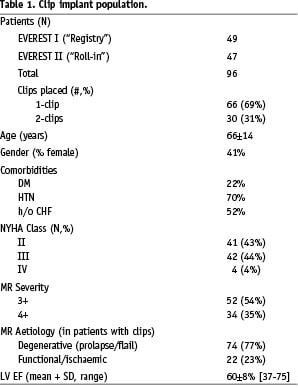
The study population was comprised of 96 patients with a mean age of 66±14 years who received 1 (66 patients) or 2 (30 patients) clips (Table 1). The aetiology of MR was degenerative disease in 77% and functional (ischemic or non-ischaemic LV dysfunction) in 23%. Other baseline characteristics shown in Table 1 include a mean LV ejection fraction of 60±8% and NYHA class III or IV in 48% of patients.
Echocardiographic parameters
The sample sizes of analysable echocardiograms available at each time point for each measured variable are shown in Table 2. The mean baseline MVA by planimetry was 6.0±1.3 cm2 and was smaller when assessed by PHT calculation (4.0±0.8 cm2). The mean MVG at baseline was 1.7±0.9 mmHg (Table 3). Following clip placement, these parameters were reassessed by transthoracic echocardiography prior to discharge (mean of 1.2±0.8 days post procedure). The mean MVA by planimetry decreased to 3.6±1.2 cm2 (p<0.05) and the mean MVG increased to 4.1±2.2 mmHg (p<0.05). From discharge to 24 months of follow-up, there were no significant changes in the mean MVA or MVG (Figure 1). The range of MVA by planimetry was 1.9 to 7.6 cm2 at discharge and 2.2 to 5.1 cm2 at 24 months.
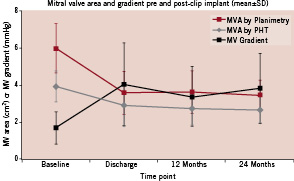
Figure 1. The mean ±SD echocardiographic MV area (cm2) by planimetry (red) and pressure half-time (PHT, in grey) and MV gradient (mmHg, in black) are shown at baseline, discharge, 12 months, and 24 months.
In order to understand the effect of residual MR on the change in MVG after clip placement, MVG at baseline and discharge were also compared in 82 patients with matched MVG data with and without acute procedural success. Acute procedural success (APS) was defined as clip implantation with MR grade <2+ on the discharge echocardiogram as determined by the core laboratory. There were no significant differences in MVG between 71 APS patients and 11 non-APS patients at baseline (1.8±0.9 mmHg vs. 1.6±0.6 mmHg, p=0.59) or at discharge (4.1±2.2 mmHg vs. 4.0±2.4 mmHg, p=0.90). Baseline MR grade in the two groups was similar (p=NS).
Subgroup analyses
Specific comparisons were made between clip patients who received 1 (69%) versus 2 clips (31%) up to 12 months. These groups were similar with respect to baseline left ventricular ejection fraction, MVA by planimetry and PHT, and MVG. The LV internal dimension during systole was slightly greater at baseline for 2-clip (37.0 mm) as compared to 1-clip (33.4 mm) patients (p=0.05). The MVA by PHT was smaller at all time points in patients with 2 clips as compared to patients with 1 clip. However, there were no significant differences between 1 and 2 clip patients in MVG or MVA by planimetry after the procedure and at 12 months (Table 3 and Figure 2).
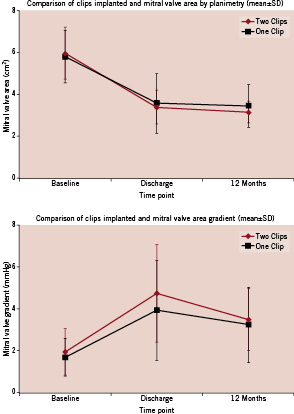
Figure 2. The mean (±SD) mitral valve area (cm2) determined by planimetry at baseline, discharge, and 12 months is compared for patients who received one clip (black) and two clips (red) in the upper panel. The mean MV gradient (mmHg) for one clip (black) and two clips (red) are compared in the lower panel at the same time points.
Comparison between 38 patients with degenerative aetiology (DMR) and 12 patients with functional aetiology (FMR) who achieved 12 month follow-up was also performed. Patients with FMR had a slightly smaller planimetered MVA at baseline (5.5±1.2 cm2 vs 6.0±1.2 cm2 in DMR patients) and post-procedure (3.3±1.7 cm2 vs 3.6±1.1 cm2), but these differences were not statistically significant (Figure 3). Mean MVG was slightly, but not statistically significantly, higher at baseline and post procedure in FMR patients as compared to DMR patients with less difference apparent over time (Table 3 and Figure 3). The decrease in MVG from discharge to 12 months was statistically significant for both DMR patients (–0.7±1.4 mmHg, p<0.05) and FMR patients (–1.3±1.7 mmHg, p<0.05), but there was no significant change in MVA by either planimetry or PHT from discharge to 12 months.
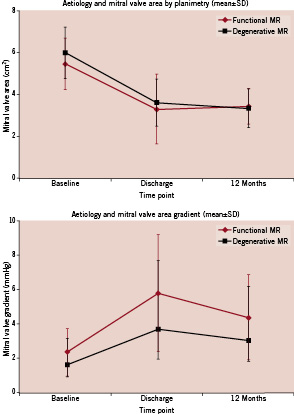
Figure 3. Patients with degenerative (black) and functional (red) aetiology of MR are compared at baseline, discharge, and 12 months with respect to mean (±SD) MV area (above) and MV gradient (below).
To further understand the effect of baseline MVA measured by planimetry on resulting valve area, a comparison was made between patients with a larger vs smaller baseline MVA (separated by the median MVA of 5.67 cm2). The median MVA in the larger orifice group was 7.0 cm2 and in the smaller orifice group was 5.0 cm2 (patients with an initial MVA < 4.0 cm2 were excluded from the trial). There were more patients with FMR in the smaller baseline orifice group (42% vs 15% in larger orifice patients, p <0.05). A larger number of patients in the larger orifice group received 2-clips (36% vs 25% in the smaller initial orifice group, p <0.05). At discharge, the MVA was smaller (3.1±0.9 cm2) in patients who started with a smaller area than in those with an initially larger area (3.9±1.2 cm2, p<0.05). However, the percentage change in MVA (about a 40% decrease) was similar for both groups. The smallest MVA observed (1.9 cm2) was in a patient who had an initial MVA of 4.4 cm2.
Discussion
The major conclusion of this study is that percutaneous repair of MR by leaflet apposition with the MitraClip® device results in an acute decrease in MVA without significant mitral obstruction and without further decrease in MVA up to 24 months of follow-up. In addition, there were no differences between patients who received one versus two clips. Patients with functional MR had a smaller MVA before and after clip placement compared to patients with degenerative disease. The percentage change in MVA was similar (about 40%) in all patients, and the smallest post-clip MVA measured was 1.9 cm2. Surgery for mitral obstruction has not been required for any patient.
Previous studies
The results of this study can be compared to surgical series examining edge-to-edge surgical repair6,18. In the largest series, Alfieri and his colleagues reported a 90% freedom from re-operation in 260 patients at 5 years6. In this report, it appeared that patients who did not receive a ring annuloplasty at the time of leaflet apposition had a greater need for re-operation during follow-up. However, annuloplasty was not a predictor of better outcome in multivariable analysis suggesting important differences between patients who received or did not receive a ring6. A subsequent report from this group examined 81 patients who underwent Alfieri repair without a ring annuloplasty18. The most common reason reported for not utilising a ring was annular calcification. Only 1 of 42 patients who did not have annular calcification, rheumatic aetiology, or underwent rescue edge-to-edge repair required re-operation at 4 years18). Other centres have also reported their results with edge-to-edge surgery. The freedom from re-operation ranged from 84% at 8 years (17) to 94% at 3 years16. Annuloplasty was performed in 56% to 88% of patients8,15-17. In general, better results were obtained in patients who underwent elective (not bail-out) edge-to-edge surgery for degenerative disease. Together, these findings suggest that excellent mid-term results can be achieved with surgical edge-to-edge repair without annuloplasty in selected patients.
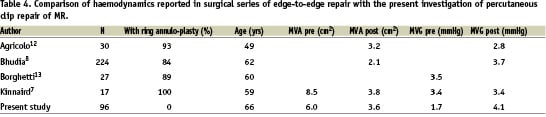
The haemodynamic effects of surgical edge-to-edge repair have also been examined7,8,11-13. In a computational model, the haemodynamic behaviour of a double orifice mitral valve was not physiologically different from a single orifice of the same total area11. This observation provides justification to estimate the overall MVA as the sum of the planimetered separate orifices and to estimate the gradient from the velocity measured through either single orifice9,10. A summary of the haemodynamics reported in surgical series is shown in Table 4. The post-operative MVA ranged from 2.1 to 3.8 cm2 with a mean MVG of 2.8 to 3.7 mmHg. The smallest post-operative MVA reported in these studies was 2.0 cm2 with no further changes over time and no patient required re-operation for mitral stenosis7,8,12,13.
The surgical results with the Alfieri operation may be compared to those recently reported in a group of patients with ischaemic MR who underwent restrictive annuloplasty and coronary revascularisation surgery19. In this study, a majority of patients had evidence for functional mitral stenosis associated with pulmonary hypertension and reduced functional capacity. However, the mean effective mitral orifice area in these patients was 1.5 cm2, substantially smaller than in both surgical series of edge-to-edge repair (Table 4) or the patients undergoing repair with the MitraClip device reported in this manuscript (3.6±1.2 cm2, Table 3).
Present investigation
The haemodynamic results of the present study are consistent with the surgical reports of edge-to-edge repair. The mean MVA by planimetry after percutaneous clip placement was 3.6 cm2 with a mean MVG of 4.1 mmHg (Tables 2, 3, 4). The average decrease in MVA by planimetry from baseline was similar, about 40%, in patients with an initially larger or smaller baseline MVA. It is important to emphasise, that patients with an initial MVA <4.0 cm2 as measured by planimetry were excluded per the protocol. Patients with functional aetiology tended to have smaller initial and post-procedure MVA and patients who received 2 clips had a slightly smaller post-procedure MVA. The smallest planimetered MVA post-clip was 1.9 cm2, and no patient required surgery or had symptoms due to mitral stenosis. There were no further decreases in MVA during up to 24 months of follow-up.
We relied primarily on the planimetered MVA and mean MVG for our conclusions. These measures have not been validated in a double orifice mitral valve, however they have been shown accurate based on a computational model11. The MVA derived from pressure half-time may be influenced by the net atrioventricular compliance and peak transmitral gradient, both of which can be affected by the presence of moderate or severe MR14. However, our conclusions relative to MVG were not changed when patients with and without acute procedural success were compared.
The pressure half-time derived MVA was smaller at all time points, including baseline, than the MVA measured by planimetry (Table 3). This finding was also observed by Kinnaird and colleagues following surgical repair7. It is possible that planimetry in the short-axis view at maximum leaflet excursion may represent the maximum orifice area whereas the PHT method may correlate better with the average mitral valve opening during diastole, potentially accounting for the discrepancy between the methods. It is also possible that the baseline planimetered mitral valve area is overestimated because the short axis plane of imaging is basal to the tips of the mitral leaflets. On the post-clip echocardiogram, the proper imaging plane for planimetry of the mitral valve is assured by clip visualisation.
Despite these limitations, the concordance of the various measures support the conclusion that the expected decrease in orifice area after repair with the MitraClip device is not associated with clinically-significant left ventricular inflow obstruction. The possibility that a modest restriction to left ventricular inflow could lead to symptoms in some patients during exercise or that mitral valve obstruction could develop during longer follow-up cannot be excluded. Two studies12,13 reported an increase in mean and peak transmitral gradients with exercise in patients following surgical edge-to-edge repair with baseline values similar to ours (Table 4). In neither study was the increase felt to be clinically significant for any patient. Furthermore, the clip does not restrict annular dynamics as observed with ring annuloplasty.
We did not observe an important difference either immediately or during follow-up between 1 clip and 2 clip patients. In part, this reflects selection bias as patients who had a small MVA after a first clip would be less likely to receive a second clip. This is supported by the observation that when patients were divided by the median initial MVA, fewer patients in the initially smaller orifice group received 2 clips. The ability to assess MVA and MVG during clip placement to determine suitability for placement of a second clip is an important feature of this procedure.
Finally, examination of the use of the MitraClip device in functional MR patients provides new conclusions. In general, patients with FMR have a worse outcome with surgery than patients with DMR20. The use of surgical edge-to-edge repair, mostly with an undersized ring annuloplasty, for functional MR has been reported7,8,15. Bhudia et al reported a higher incidence of recurrent MR in FMR patients as compared to patients with degenerative aetiology8. However, the edge-to-edge repair was primarily used as a bail-out technique after failed partial ring annuloplasty in his series. DeBonis et al reported significantly lower MR recurrence over time in patients with intent to treat edge-to-edge repair and an undersized annuloplasty ring as compared to patients with an undersized ring alone15. The results of percutaneous repair for FMR reduction were not reported in this study, but appear comparable to the results in DMR patients in a preliminary analysis21. In this study, we did not observe any important differences between FMR and DMR patients in MVA by planimetry or PHT or MVG at 12 months of follow-up. The MVA that we report after percutaneous repair is substantially larger than that reported after restrictive surgical annuloplasty for FMR19, and is similar to previous surgical edge-to-edge repair series shown in Table 4.
In summary, our findings demonstrate an expected decrease in MVA after percutaneous clip repair without further changes in MVA for up to 2 years. No patient required surgery for left ventricular inflow obstruction, and the results were not influenced by the use of more than 1 clip or the MR aetiology.

