Abstract
Aims: To validate and test in vivo a new modality of quantitative coronary angiography (QCA), dual QCA (D-QCA), developed to quantify intracoronary thrombotic burden (ITB).
Methods and results: Calculation of ITB with D-QCA is based on the discrepancy of luminal areas assessed with edge detection (ED) and video-densitometry (VD), measured with Cardiovascular Angiography Analysis System II. Experimental validation was first performed in phantoms with known obstructive volumes. In vivo assessment of thrombotic burden changes was performed in angiograms from 19 patients with large ITB, obtained before and after antithrombotic treatment, and compared with semi-quantitative assessment (TIMI thrombus grade (TTG)).
A good correlation between D-QCA and true occlusive volumes was found (y=9.21+0.99x, r=0.996). Intra- and inter-observer variability was 2.77±10.97 mm3 (p=0.50) and –1.28±6.99 mm3 (p=0.62) respectively. In vivo, D-QCA demonstrated a significant reduction in ITB resulting from treatment (137.22±120.13 mm3 before and 104.72±99.19 mm3 after treatment, p=0.001). Overall, TTG also decreased (3.63±0.68 before and 3.11±1.20 after, p=0.008), but in those nine (47%) patients in which remained unchanged D-QCA detected a reduction in ITB (pre 148.17±154.03 mm3, post 112.86±117.82 mm3, p=0.05).
Conclusions: D-QCA appears as a useful approach to quantify IC thrombus volume, being more sensitive than TTG in assessing changes in ITB resulting from treatment strategies.
Introduction
Despite current available techniques, percutaneous treatment of coronary stenoses with large intracoronary thrombus has been associated with increased complications, such as no-reflow phenomenon, distal embolisation and acute stent thrombosis after percutaneous coronary intervention and therefore an increase in short-term mortality1-6. The efficacy of mechanical or pharmacological treatments aimed to reduce thrombotic burden cannot be assessed directly, since a methodology to quantify thrombus burden is not available.
Quantitative coronary angiography (QCA) based on edge detection (ED) or video-densitometry (VD) has been extensively validated previously for assessing coronary stenosis severity7-10, but not to quantify thrombotic burden. In this study, we explore the use of a novel approach, based on the combined use of ED and VD modalities, to quantify intracoronary thrombosis with QCA. The study was performed first in radiological phantoms to validate the technique, and then on serial coronary angiograms of patients with large intracoronary thrombus undergoing antithrombotic treatment.
Methods
The technique used in this study was a combination of edge detection (ED) and video-densitometry (VD) QCA modalities. For its validation, it was first tested in vitro using angiographic phantoms, and then applied in a subset of coronary angiograms obtained in patients with large intracoronary thrombotic burden who were undergoing antithrombotic treatment in an attempt to reduce its volume.
Quantitative coronary angiography and combined analysis of VD and ED area functions
Quantitative coronary angiography image analysis was performed using the Cardiovascular Angiography Analysis System II (CAAS II system, Pie Medical, Maastricht, Netherlands) which has been validated previously in studies for both ED and VD features11,12. The system was first calibrated using the guiding catheter as a scaling device. After identification of the proximal and distal segments to the stenosis, a centreline was automatically drawn by the QCA system. Edge detection of luminal borders was then automatically performed based on the brightness profile (pixels) of individual scan lines generated perpendicularly to the vessel centreline. At the site of coronary thrombus, luminal edges were manually traced by the operator following the expected contour without thrombus or stenosis. Luminal densitometric area measurements within the segment were automatically calculated by the system by converting the density profile of each individual scan line perpendicular to the vessel centreline to absolute area value9. Transformation of density to area units was performed based on the video-densitometric profile of the neighbour, non-stenotic segment, which was used by the system as a reference and in which luminal area was calculated geometrically using the vessel diameter (ED) and assuming the luminal shape was circular. The estimated VD and ED areas, expressed in mm2, are then represented along the analysed segment (mm) as area functions within a single graph (Figure 1). Calculation of thrombus volume was performed using a built-in-volumetric tool incorporated in the CAAS II system, which calculates obstructive volume (mm3) based on the difference between ED and VD area functions along the analysed segment.
Validation with stenosis phantoms
Stenosis phantoms (Figure 1) were built using eight segments of 9 mm outer diameter (7 mm inner diameter) polyethylene tube. Each tube segment contained a simulated thrombus made from play-doh (Hasbro, United Kingdom) that was rolled into a 4 mm diameter cylinder and cut into 8 different lengths (5, 10, 15, 20, 25, 40, 45, 50 mm) using a micrometer. Before being placed into the polyethylene tube, each simulated thrombus was dropped into a micrometer cylinder filled with water and the resulting volume displacement was recorded as equivalent to thrombus volume. A total of 8 phantoms were built, five concentric (5, 10, 15, 20, 25 mm) and three eccentric (40, 45, 50 mm). The eccentric simulated thrombi were made by carefully deforming their shape within the tube to simulate long irregular mural thrombi. Phantoms were then filled with iodinated angiographic contrast (Lopamiro 370 [75.5 g/ml], BRACCO, Turin, Italy) and placed separately on the angiographic table on top of lead filtering (1 mm). A 6 Fr Judkins left 4 guiding catheter (Cordis, Johnson & Johnson, Warren, NJ, USA), filled with contrast, was placed next to the phantom to be used as a scaling device. Image acquisitions of the each stenosis phantom in anterior-posterior projection were acquired using a Philips Inturis angiographic system (Philips, Eindhoven, The Netherlands) with the distance between the X-ray tube and all the stenosis phantoms being kept constant. Each image was analysed off-line by two independent observers using the CAAS II system (dual QCA) to calculate the described obstructive volume (mm3). The correlation between the obtained mean measurements and the actual volume of the simulated thrombus was obtained using linear regression analysis.
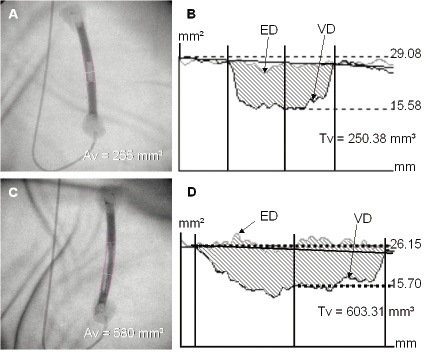
Figure 1. Dual QCA on a concentric and an eccentric stenosis phantom. Angiographic images are shown of both a concentric stenosis phantom (Figure 1a) and an eccentric stenosis phantom (Figure 1c) with their corresponding graphical plots (Figure 1b and Figure 1d) of edge detection (ED) and video-densitometry (VD) functions. Actual obstructive volume (Av) (mm3) and QCA derived obstructive volume (Tv) (mm3) are also displayed.
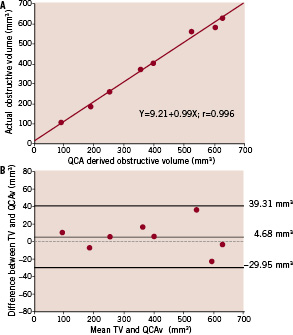
Figure 2. a: Correlation between actual obstructive volume (Tv) and QCA derived obstructive volume (QCAv) for the stenosis phantoms. 2b: Bland-Altman plot displaying the difference in Tv and QCAv against the average of Tv and QCAv. Upper and lower limits correspond to mean±1.96 standard deviation.
In vivo assessment of thrombus burden
Once the system was validated, the approach was used to analyse the pre- and post- angiograms of patients with large intracoronary thrombus (Figure 3) with at least TIMI 1 flow before and after treatment with antithrombotic regimen of aspirin, clopidogrel, heparin and glycoprotein IIb/IIIa inhibitors. Angiographic projections without foreshortening and vessel overlap were selected and dual QCA was applied to measure the volume of thrombus pre- and post- treatment. Two independent observers performed the method of Dual QCA provided by the CAAS II system to assess intra- and inter- observer variability. Pre- and post- mean values for both observers were then compared in order to detect any significant difference in thrombus volume with the use of antithrombotic therapy. Semi-quantitative assessment by two independent observers (to assess intra- and inter- observer variability) using the TIMI thrombus grade13 pre- and post-treatment were also measured and compared to detect any changes. Particular attention was paid to those patients in whom TIMI thrombus grade remained unchanged after treatment.
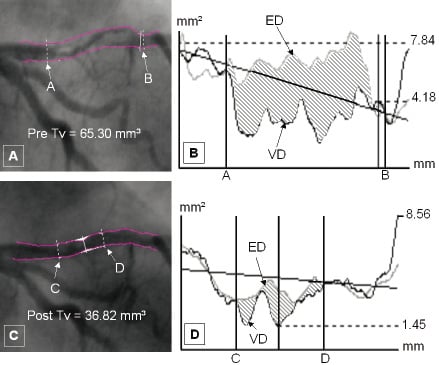
Figure 3. Dual QCA analysis applied to a stenosis containing large intracoronary thrombus in the proximal left anterior descending artery before and after antithrombotic therapy. Figures 3a and 3c show the stenosis pre- and post- treatment with the luminal edges reconstructed and the thrombus borders demarcated using CAAS-II system. Figures 3b and 3d show the corresponding area plots pre- and post- treatment respectively, with the thrombus borders defined by the solid outer lines. The shaded area corresponds to the mismatch between edge detection (ED) and video-densitometry (VD) derived luminal areas in both graphs caused by intracoronary thrombus. Tv: Thrombus volume
Statistical analysis
Statistical analysis was performed using the statistical program StatView (SAS Institute Inc, Cary, NC, USA). Mean and standard deviations were used for continuous variables while percentages were used for the discrete variables. Linear correlation analysis was applied using a least square method to identify linear correlation between the calculated and true volumes of the stenosis phantoms. The Bland-Altman analysis was used to assess the degree of agreement between the calculated and true volumes of the stenosis phantoms. Comparison of means between groups was performed by applying the Student´s t test for paired data. The Bland-Altman analysis was also used for intra- and inter-observer variability. The statistical level of significance was fixed at a 5% level.
Results
In vitro phantoms measurements
Comparison between QCA-derived (QCAv) and actual obstructive volume (Tv) demonstrated that QCA-derived method has an excellent correlation and does provide a very good estimation of the actual obstructive volume (p=0.48). The regression equation obtained was Tv=9.21+0.99 x QCAv with a correlation coefficient of 0.996 (Figure 2a). Mean difference between the QCA-derived method and the actual volume was 4.68±17.67 mm3 (p=0.48) (Figure 2b) while the mean intra- and inter- observer differences for the method were found to be extremely low at 2.77±10.97 mm3 (p=0.50) (Figure 5a) and –1.28±6.99 mm3 (p=0.62) (Figure 5b) respectively.
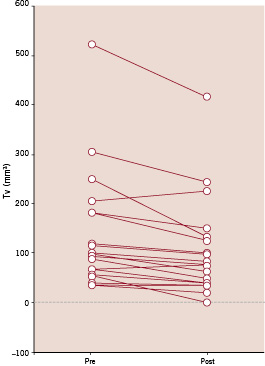
Figure 4. Individual changes in thrombotic burden as a result of antithrombotic treatment assessed with dual QCA. Tv=thrombus volume.
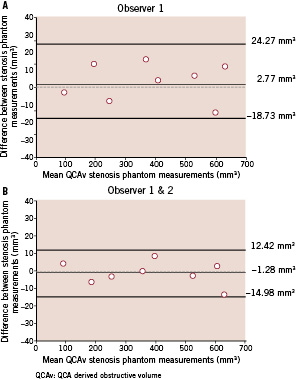
Figure 5. Intra-observer (a) and inter-observer variability (b) for the stenosis phantoms are shown graphically using the Bland-Altman plots.
In vivo coronary measurements
The demographic data of the 19 patients in whom dual QCA was performed before and after antithrombotic treatment on stenoses are shown in Table 1. Relevant angiographic data is shown in Table 2. A significant change in absolute thrombus volume (pre 137.22±120.13 mm3, post 104.72±99.19 mm3, p=0.001) was documented using dual QCA, with a mean reduction of 26% (Table 2 and Figure 4). Intra-observer differences in measurements were -0.86±9.89 mm3 before (Figure 6a) and 0.71±10.77 mm3 (Figure 6b) after antithrombotic treatment (non-significant in both cases). Inter-observer differences were 2.83±12.21 mm3 before (Figure 6c) and 1.09±11.27 mm3 (Figure 6d) after antithrombotic treatment.
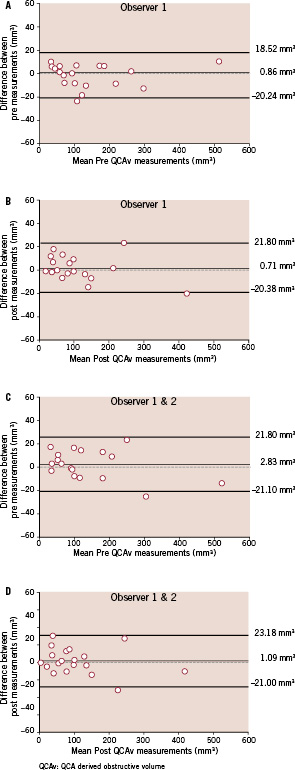
Figure 6 (a-d). Intra-observer (a-b) and inter-observer (c-d) variability before and after the use of antithrombotic treatment are shown graphically using the Bland-Altman plots.
In terms of TIMI thrombus grade, a significant reduction of 17% (3.63±0.68 before and 3.11±1.20 after, p=0.008) was observed which was consistent with the change observed with the method of dual QCA (Table 2). However, despite an overall change in TIMI thrombus grade, no change was noted in nine patients. In this subgroup of nine patients, dual QCA revealed a decrease in mean thrombus volume (pre 148.17±154.03 mm3, post 112.86 ±117.82 mm3, p=0.05) that was not detected by the visual assessment, indicating a superiority of QCA-derived method over semi-quantitative assessment in assessing changes in thrombus volume.
Discussion
The main findings of our study are: 1) dual QCA combining both edge detection and video-densitometry constitutes an alternative, feasible approach to quantify intracoronary thrombus burden from coronary angiograms; and 2) dual QCA is more sensitive than TIMI thrombus grading in detecting changes in intracoronary thrombus volume resulting from pharmacological treatment.
The results of this work may contribute to the solution of a pending problem: the quantification of intracoronary thrombotic burden. In spite of the importance of this key feature of acute coronary syndromes, no quantitative method of measuring in vivo intra-coronary thrombus volume was available. Assessment of therapeutic strategies in this context has been based either on the recording of clinical variables (such as major adverse cardiovascular events), or on indirect changes in coronary angiography (TIMI grade flow and myocardial blush grade) or ECG (ST elevation). The only attempt to quantify thrombus has been the semi-quantitative TIMI thrombus grading scale. This scale has been used in studies in which pharmacological modification of intracoronary thrombus was tested, like the PRISM-PLUS trial, in which 23% significant reduction in thrombus burden was documented with this method. Although this figure is in apparent agreement with the findings in this study, it has to be stressed that the inclusion of patients treated with antithrombotic agents in our work was aimed solely to test the ability of the technique to identify changes in thrombotic burden, and not to demonstrate or quantify its efficacy.
The present research constitutes a first attempt to use widely available QCA features to quantify intracoronary thrombosis. The use of video-densitometry as an alternative to edge detection QCA has been explored previously on the grounds of its potential to assess luminal area irrespectively of the angiographic projection used14,15, but not as a synergistic technique to edge detection. For the purpose of this study, angiographic phantoms were used in the first place to validate the potential use of these techniques in simulated thrombi. The results showed an excellent correlation, providing a regression equation that allowed calculation of the actual thrombus volume. Repeated readings by 2 observers demonstrated a low intra- and inter- observer variability. More importantly, when applied in the less favourable in vivo conditions, in which angiographic images of vessels with large intracoronary thrombus pose the problem of superposition of other radiological structures like bone, air, etc., the method was able to detect changes in thrombotic burden secondary to antithrombotic drug treatment which were below the sensitivity of semi-quantitative grading. The overall 26% reduction in thrombotic burden documented in our study population using dual QCA might at first glance be similar to that documented with TIMI thrombus grading in the PRISM-PLUS trial13. However, a higher sensitivity of dual QCA can be inferred from the measurements performed in the important subgroup (47%) of vessels without apparent change in TIMI thrombus grading.
The described method of assessing thrombus volume provides an attractive approach for future assessment of different treatment strategies on thrombus containing stenosis. Besides the limitations inherent to angiography, there are limitations to its application, some of them derived from being a first study of its kind. The method appears suitable only to vessels with at least TIMI I flow, in order to define the luminal borders and the extension of the thrombotic process. Tracing of vessel edges was performed manually by extrapolation of neighbour luminal segments without filling defects. Although intra- and inter- observer variability was low, suggesting that even in our experimental setting measurements can be safely repeated, there is an unavoidable intra- and inter- observer error in reconstruction of the vessel. Another limitation of our approach, common to all angiographic techniques, is that it is not possible to distinguish between protruding atheroma and intraluminal thrombus, and that therefore the volume obtained by the method represents a combine plaque and thrombus volume.
With all these limitations in mind, we believe that the results of this research demonstrate that dual QCA is a simple and safe method which makes possible a richer use of the information contained in coronary angiograms in which the clinician suspects that intraluminal defects are likely to be of thrombotic origin. Further research will demonstrate its potential as a research tool; such as in the assessment of intracoronary thrombus extraction devices, drug treatments addressed to reduce thrombotic burden, and other therapeutic strategies.

