Abstract
Aims: Diabetes mellitus (DM) plays an important role in the development of coronary artery disease. Although previous studies have associated drug-eluting stent (DES) implantation in diabetic patients with favourable clinical and angiographic outcomes, the very long-term efficacy of these devices in diabetic patients undergoing PCI for significant unprotected left main coronary artery (ULMCA) disease has not been established yet.
Methods and results: Consecutive diabetic patients (n=100), who underwent elective PCI with DES for de novo lesions in an ULMCA between April 2002 and April 2004 in seven tertiary health care centres, were identified retrospectively and analysed. Consecutive non-diabetic patients (n=193), who underwent elective DES implantation for unprotected ULMCA disease, were selected as a control group. All patients were followed for at least 36 months. At 3-years follow-up, freedom from cardiac death & myocardial infarction (CDMI), target lesion revascularisation (TLR) and target vessel revascularisation (TVR) did not differ significantly between groups. The adjusted freedom from major adverse cardiac events (MACE, defined as the occurrence of CD, MI or TVR) was 63.4% in the DM group and 77.6% in the controls (p<0.001). When divided into IDDM and NIDDM sub-groups, insulin-dependent DM (IDDM) but not non IDDM (NIDDM) patients had significantly lower freedom from CDMI, TLR, TVR and MACE compared to controls.
Conclusions: These results suggest that major improvements in DES technology and pharmacotherapy are still required to improve clinical outcome and that the decision to perform percutaneous revascularisation in this subset of patients should be taken cautiously and on a case by case basis.
Introduction
Diabetes mellitus negatively impacts clinical outcome in patients undergoing surgical or percutaneous arterial revascularisation1-3. Proposed explanations for this increased vulnerability include greater atherosclerotic plaque burden, longer lesion length and aberrant neointimal proliferation after stenting in diabetics. Several trials conducted in diabetic patients determined that percutaneous coronary intervention (PCI) with drug-eluting stents (DES) significantly reduced restenosis, resulting in superior long-term clinical and angiographic results, when compared with bare metal stents (BMS)4,5. However, whether PCI with DES is a safe and effective alternative to CABG remains a matter of debate6-9. Importantly, there is little data that addresses clinical outcome associated with DES use to treat unprotected left main coronary artery (ULMCA) disease in diabetic patients, and studies reporting very-long term data are notably lacking.
Thus, the aim of this study was to report for the first time, very-long term clinical outcome data for diabetic patients undergoing PCI to ULMCA lesions with DES, and to identify potential predictors of adverse events using an international, multicentre, retrospective registry design.
Methods
Population
Consecutive diabetic patients who underwent elective PCI with SES or PES for de novo lesions in an ULMCA between April 2002 and April 2004 in seven European and US tertiary health care centres, were identified retrospectively and analysed. Patients were eligible for inclusion if they were undergoing pharmacological treatment with either hypoglycaemic agents or insulin at the time of the index procedure. Patients with acute myocardial infarction or cardiogenic shock at the time of admission were excluded from the analysis. Consecutive non-diabetic patients, who underwent elective DES implantation for unprotected ULMCA disease at the same centres over the same time period, were selected as a control group. All patients had confirmed myocardial ischaemia related to ULMCA disease. Factors that determined choice of a percutaneous approach over surgery included coronary anatomy and lesion characteristics, patient preference, referring physician preference and surgical risk. Patients were stratified by risk class using the European System for Cardiac Operative Risk Evaluation (EuroSCORE) (Table 1).
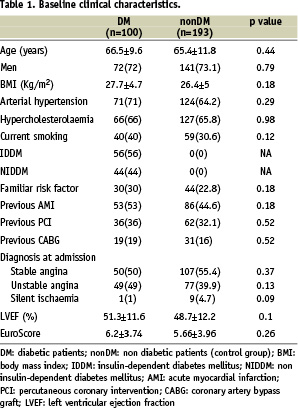
Subjects with a EuroSCORE >6 were defined as high-risk and those with a EuroSCORE >9 as very high-risk. Data analysis was performed with the approval of the institutional ethics committees of the hospitals and/or universities involved.
Procedures and medications
All PCIs were performed according to current guidelines. Route of arterial access, type of stent, stenting strategy, use of periprocedural glycoprotein IIb/IIIa inhibitors, intravascular ultrasound guidance and prophylactic intra-aortic balloon pump use was at the discretion of the operator.
All patients received 325mg of aspirin, clopidogrel (75 mg/day three days prior to the procedure or 300-mg loading dose) and low-molecular-weight or unfractioned heparin titrated to maintain an activated clotting time >250 s. Complete revascularisation was attempted in all patients at the time of the index PCI. Significant lesions that were not treated during the index procedure were staged and treated generally within one month. After the procedure, all patients were prescribed lifelong aspirin (75-100 mg/day) and prolonged (at least six months) dual antiplatelet therapy (DAT) of aspirin 100-325 mg/d and 75 mg/d clopidogrel or 250 mg ticlopidine twice daily. Repeat revascularisation was only performed if there was either symptom recurrence or inducible ischemia related to the ULMCA disease.
Definitions
Technical success was defined as successful deployment of a stent(s) in the target lesion.
Procedural success was defined as ULMCA revascularisation with < 30% residual diameter stenosis by quantitative coronary angiography, without major procedural or post-procedural adverse events (death, myocardial infarction, emergency target vessel revascularisation or acute stent thrombosis).
Death was classified as either cardiac (CD) or non-cardiac, according to the Academic Research Consortium (ARC) definition10. Deaths that could not be classified were considered cardiac.
Target lesion revascularisation (TLR) was defined as any repeat percutaneous intervention of the target lesion performed for restenosis or other complication of the target lesion. The target lesion was defined as the treated segment from 5 mm proximal to the stent to 5 mm distal to the stent.
Target vessel revascularisation (TVR) was defined as any repeat PCI of any segment of the target vessel, defined as the entire major coronary vessel proximal and distal to the target lesion, including upstream and downstream branches and the target lesion itself. In the context of LMCA disease, the TVR definition comprises the whole left coronary system.
Major adverse cardiac event (MACE) was defined as the occurrence of cardiac death (CD), nonfatal myocardial infarction (MI) or TVR during the follow-up period.
Definite, probable and possible stent thromboses were determined according to the ARC definitions. Stent thrombosis was defined as acute, sub-acute, late and very late if the event occurred within 24 hr, 30 days, <1 year or >1 year respectively, after the procedure.
Myocardial infarction was defined as creatine kinase-MB mass increase >3 times the upper limit of normal, associated with chest pain lasting >30 min or with new evident electrocardiographic changes.
Data collection, and follow-up
Information regarding clinical status was collected at clinic visits and by telephone interview scheduled 30 days after the procedure and then every six months. When the patient was not reachable, information were gathered from the referring physician, hospital electronic database or Municipal Civil Registries. The data collection was carried out using a dedicated electronic case report form (CRF). All the explored variables in the CRF were defined and number-coded before the CRF was sent to each participating centre. At three years the clinical follow-up was 100%. This protocol was approved by the hospital ethics committees and is in accordance with the Declaration of Helsinki. Written informed consent was obtained from every patient.
Statistical analysis
Normally distributed variables were analysed using parametric tests and non-normally distributed data using non-parametric tests. Continuous variables are expressed as mean ±SD and differences were compared using Student t test. Categorical variables are expressed as counts and percentages. Differences between sub-groups were assessed by Fisher exact test or chi-square test, as appropriate. Bivariate and multiple variable analyses were performed to identify independent predictors of adverse events. Specifically, all variables significantly associated with the clinical event of interest on bivariate analysis (p<0.10) were entered into subsequent models. After appropriate checks for underlying assumptions, multivariate Cox proportional hazard analysis was then performed, with the enter method for all pertinent covariates. Results of multivariate Cox analyses are reported as hazard ratios (HR) with 95% confidence intervals and p values. MACEs were reported hierarchically. Survival curves were generated at mean of covariates and differences between groups were evaluated and reported using hazard ratios (HRs) with 95% CI and p values. Landmark analysis was performed with the landmark set at 12 months to provide separate descriptions of the early and late relative risk of MACE in the SES and PES sub-groups. Outcomes in the two sub-groups were compared using risk ratios with 95% CIs. All analyses were performed using SPSS version 12 statistical software (SPSS Inc., Chicago, IL, USA). A two-tailed p value < 0.05 was considered significant for hypothesis testing.
Results
Baseline clinical, procedural and angiographic characteristics
Baseline characteristics are shown in Table 1 and 2.
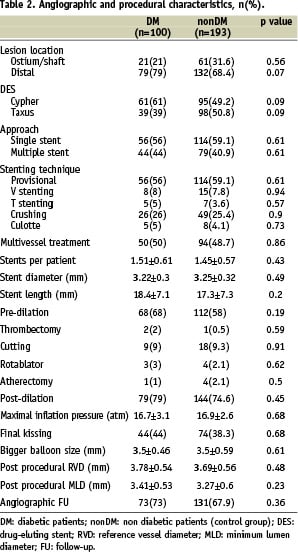
Baseline characteristics were comparable in the study population (n=100) and control group (n=193). The majority of patients were male, slightly overweight, with hypertension and/or hypercholesterolemia. Nearly half of the population had had a previous MI and were admitted to the hospital with the diagnosis of stable angina. Among DM patients, 56% had IDDM and mean EuroSCORE was 6.2±3.74. In both groups, approximately 75% of all lesions were distal and 40% of these were treated with a double stent approach. The favoured stenting technique was crush stenting. During the same period, a total of 680 patients with significant ULMCA disease underwent CABG in the seven participating centres.
Follow-up clinical outcomes
Short and long-term clinical outcomes are summarised in Table 3.
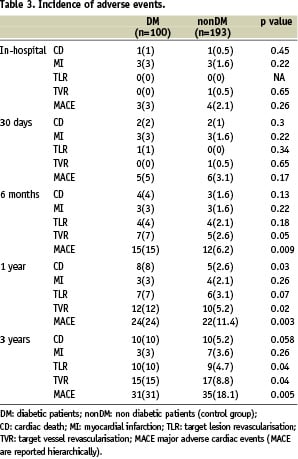
All patients were followed for at least 36 months (range: from 36 to 57). No major differences in clinical outcome were found between the two groups at 30 days FU. Conversely, at 1-year, the incidence of CD, TLR, TVR and MACE was significantly higher in the DM group, compared to the controls. At 3-years FU, the incidence of overall death was 13% in the DM group and 7.2% in the non DM group (p=0.04). The incidence of TLR, TVR and MACE remained significantly higher in the study group but CD failed to reach statistical significance (p=0.058).
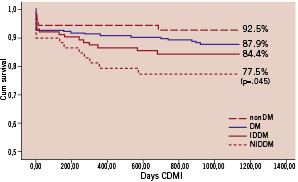
Adjusted curves derived from Cox survival analysis are displayed in Figure 1 (panels A-D).
Panel A: Freedom from CDMI
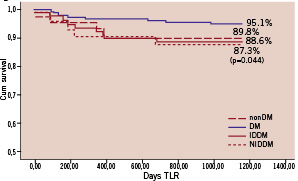
Panel B: Freedom from TLR
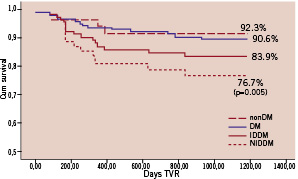
Panel C: Freedom from TVR
Panel D: Freedom from MACE and landmark analysis
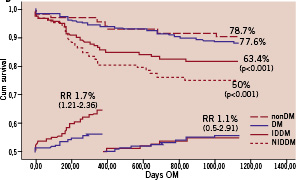
Figure 1 (panels A-D). Freedom from CDMI, TLR, TVR and MACE at means of covariates. The nonDM subgroup was taken as reference group. P values, derived from the comparison between each subgroup with nonDM, are shown only when statistical significance was reached.
Freedom from MACE was 63.4% in the DM group and 77.6% in the controls (p<0.001) but freedom from CD&MI, TLR and TVR did not differ significantly between groups.
When divided into IDDM and NIDDM sub-groups, IDDM but not NIDDM patients had significantly lower freedom from CDMI, TLR, TVR and MACE compared to controls.
Multiple variable analyses
Results of multiple variable analyses are presented in Table 4.
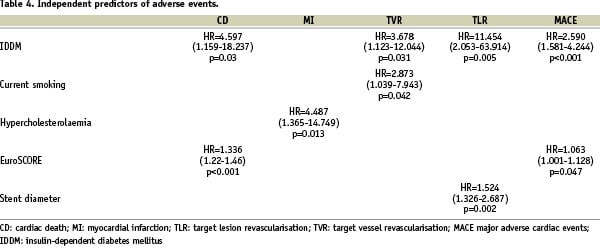
Of note, IDDM was identified as an independent predictor for CD, TVR, TLR and MACE. EuroSCORE predicted CD and MACE, and hypercholesterolaemia, current smoking and stent diameter predicted MI, TVR and TLR, respectively.
Discussion
The main findings of the present study are the following: 1) long-term freedom from CD and MI of diabetic patients undergoing PCI for ULMCA disease are for the first time reported; 2) diabetic patients had a significantly higher incidence of MACE, compared to non-DM patients and this primarily reflects a higher TVR rate; 3) the majority of adverse events occurred within the first year and thereafter the event rate tended to stabilise over time; 4) IDDM patients had significantly worse clinical outcome compared to NIDDM and non-DM patients.
Diabetes mellitus plays a major role in the development and progression of coronary artery disease. Proposed mechanisms to explain the increased severity of CAD and poorer outcomes after PCIs in diabetic patients include a heightened inflammatory response, endothelial dysfunction, increased plaque burden and altered coagulation processes11-14. Introduction of DES has dramatically reduced the rate of stent restenosis and broadened the indications for PCI. This advance has shifted many subsets of patients with complex angiographic disease into the realm of percutaneous revascularisation and away from CABG. Previous large studies of diabetic patients have associated DES implantation with favourable mid-term clinical and angiographic results2,15-17. However, the impact of DES on very long-term clinical outcome is still largely unknown. Furthermore, the efficacy of these devices in diabetic patients undergoing PCI for significant ULMCA disease has not been established.
For the first time, the present results indicate that DES implantation in diabetic patients with ULMCA disease is feasible and effective over time. Notwithstanding the higher risk profile of our cohort (mean EuroSCORE 6.2±3.74), freedom from CD and MI was comparable with that of a previously reported study in diabetics with other subsets of lesions18,19. Consistent with these results, freedom from TLR in the DM patients was not significantly different from the control group, confirming the results of previous IVUS studies in which DES led to similar results both in DM and non-DM patients20,21. The presence of left main coronary artery disease, especially when it is not associated with other vessel disease, does not appear to increase the risk of adverse events. We speculate that large vessel size together with prolonged dual antiplatelet therapy that is usually prescribed to these patients may partially mitigate adverse responses previously determined in these patients after PCI.
Diabetic patients had significantly higher 1-year MACE rates compared with non diabetic patients. This was mostly driven by a higher rate of TVR. These results are in line with the ARTS II diabetic sub-study findings22 and confirm the tendency in this subset of patients to develop new lesions and to have faster progression and more complex patterns of disease23,24.
Previous studies have associated paclitaxel-eluting stents with a significant reduction in MACE in diabetic patients when compared to sirolimus. This advantage was hypothesised to be related to the different pathways targeted by the two compounds, which, in the case of paclitaxel was not affected by insulin-resistance25. Although in our study the sample size for this comparison is small, when tested in the multivariable model, SES and PES did not differ in efficacy. Although the “aggressiveness” of CAD in diabetic patients is indisputable, the relatively high incidence of MACE in this population could be also a matter of definitions. In fact, it is worth noting that in the context of LMCA disease, the TVR definition comprises the whole left coronary system and therefore the likelihood of a re-intervention due to a new lesion is much higher in this subset when compared to populations with multivessel disease.
The survival curves showed that adverse events mainly occurred within the first 12 months, while thereafter the curves progressively flattened. This tendency was particularly evident in the DM group, in which 77.4% of all MACEs occurred within the first year of follow-up. Moreover, the landmark analysis showed a significantly increased risk ratio for incidence of MACE in the DM patients relative to the non-DM group at one year. Our results, though underpowered to detect these differences, are consistent with findings in previous studies that identify diabetes as a strong independent predictor of progression of atheroma burden23,24. Based on our findings, diabetic patients with ULMCA disease undergoing PCI with DES implantation should be followed closely, and especially during the first year after the procedure.
In the present study, adverse outcome comprising CDMI, TLR, TVR as well as MACE was significantly increased in diabetics treated with insulin. In contrast, NIDDM patients had clinical outcomes that were not significantly different from the control group. This finding, which is consistent with prior reports19,26-28, has several potential explanations. These include direct effects of exogenous insulin on neointimal proliferation and expression on IL-6 and TNF29,30. Furthermore, type II diabetic patients may have more severe cardiovascular disease at the time when insulin treatment is instituted31,32.
At 3-year follow up, 50% of IDDM patients experienced an adverse event. In IDDM patients that underwent surgical revascularisation, the 5-year incidence of death was 12.2%33 while the incidence of MACE was 25%34. Though limited, these results suggest that major improvements in DES technology and pharmacotherapy are still required to improve clinical outcome and that the decision to perform percutaneous revascularisation in this subset of patients should be decided cautiously and on a case by case basis.
Limitations
The present study was designed as a retrospective multicentre registry and therefore lacks randomisation and intention-to-treat data. Since no sample size calculations were performed, we acknowledge that our results may be affected by a type II error. Moreover, information on HbA1c is missing and its potential impact on clinical outcomes could not be evaluated.

