Abstract
Optical coherence tomography (OCT) has become a key intracoronary imaging modality able to traverse some of the limitations of angiography and intravascular ultrasound. In vivo imaging with high resolution (around 15 micrometres) has given unique insights into not only atherosclerotic plaque, but also to the understanding of tissue responses underlying stent implantation. Novel developments with faster OCT pullback speeds will further simplify the procedural requirements and eventually eliminate the need for proximal vessel balloon occlusion during image acquisition. This report explores the current and future developments in OCT technology that will see this unique imaging modality become a key player in both the clinical and research arena for the interventional cardiologist.
Introduction
The application of optical coherence tomography (OCT) to the coronary arteries has offered new insights into atherosclerosis and tissue responses following stent implantation. The clear and detailed images have generated an intense interest in adopting this technique for both clinical and research purposes. The first OCT system for coronary imaging was approved for clinical use in the European Union in 2004 and, up to now, more than 100 systems have been installed in catheterisation laboratories world-wide. OCT has been used for the detailed assessment of atherosclerotic plaque, in particular the quantification of thin-cap fibroatheroma (TCFA) and the distribution of macrophages (foam cells) surrounding vulnerable plaques.1-6 In addition, OCT can precisely assess tissue coverage following stent implantation,7-12 and in this regard, may provide greater insights into the potential link between stent endothelialisation and late thrombosis. This report details current and future developments in OCT imaging, which include exciting technological advancements that will consolidate the position of OCT as a key diagnostic tool to complement the armamentarium of the cardiologist well into the future.
Current OCT technology
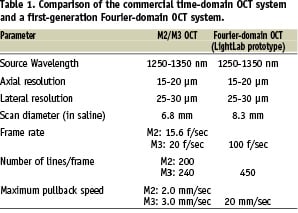
At present, two commercial OCT systems, the M2 and M3 OCT Imaging Systems (LightLab Imaging, Inc., Westford, MA, USA), are available for clinical use. Both employ a dedicated fibre-optic imaging wire (ImageWireTM, LightLab Imaging Inc., Westford, MA, USA) connected to a patient interface unit (PIU) which, in turn, connects to the system console. The imaging wire has a maximum outer diameter of 0.019” (with a standard 0.014» radiopaque coiled tip) and contains a single-mode fibre-optic core that rotates within a transparent sheath. Figure 1 illustrates the M2 system console with the cart containing the optical imaging engine and the computer. The mouse, keyboard, two monitors, and the PIU are all mounted on top of the cart. Although both the M2 and M3 systems use the identical console and PC-based operating system, the latter permits imaging at a higher frame rate (20/sec for the M3 vs. 15.6/sec for the M2) with a faster maximum pullback speed (3.0mm/sec vs. 2.0 mm/sec).

Figure 1. The LightLab M2 System console.
OCT physics
Current OCT systems incorporate advanced near-infrared light sources and optical components that operate in a wavelength band centred on 1310 nm. Like intravascular ultrasound (IVUS), OCT measures the depth of reflections from tissue according to the round-trip propagation time of reflected energy. However, as the speed of light is much faster than that of sound, an interferometer is required to measure the backscattered light. The interferometer splits the light from the source into two paths a reference path that directs the light to a reference moving mirror and a sample path that directs light through the fibre-optic imaging wire into the tissue and collects the backscattered light. The light that returns from both paths is recombined at a detector, generating the so-called interferogram from the sum of the reference and sample fields (Figure 2a). The maximum imaging depth of current OCT systems is 1.0-2.0 mm, depending on tissue type, with axial and lateral resolutions of 15 and 25 µm, respectively.
The time-domain OCT imaging method on which the M2 and M3 systems are based relies on a moving mirror to scan each depth position in the image pixel by pixel. This mechanical scanning process limits the rate at which images can be acquired. The latest generation of OCT systems employ Fourier-domain OCT acquisition methods, described in a later section of this report, that enable much faster image acquisition rates and pullback speeds.
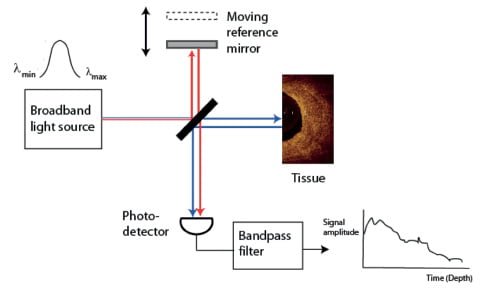
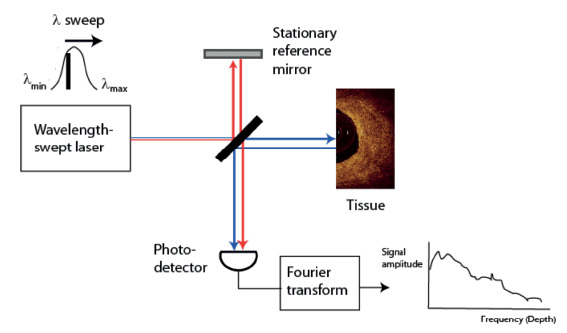
Figure 2. Main components of (a) time-domain and (b) Fourier-domain OCT systems. The red and blue lines depict the sample and reference beams, respectively, that generate the interference signal at the photo detector.
Procedural requirements for OCT imaging
As near-infrared light penetrates only a short distance through blood, temporary blood clearance is required for OCT imaging. To clear blood according to the instructions for use of the LightLab M2 system, a proximal occlusion balloon (Helios, Goodman Co. Ltd., Nagoya, Japan) is inflated to between 0.5-0.7 atm, while simultaneously flushing physiological saline or Ringer’s lactate solution through the distal lumen of the balloon catheter at a rate of 0.5–1 ml/s. Refinements in OCT technology have led to faster acquisition speeds and have encouraged a gradual shift from the cumbersome occlusive technique to a simpler, non-occlusive approach with flushing of viscous contrast through the guiding catheter. Even though initial reports have shown this to be feasible,13 this technique is still regarded as “off-label” although, intrinsically, it has a number of advantages and eliminates the risk of balloon-related vessel injury.
Fourier-domain OCT
Principles
The development of a new generation of OCT systems with faster image acquisition speeds and greater scan depths, without loss of vital detail and resolution, is a major advancement in intravascular OCT imaging. Rather than using a broadband light source as in conventional time-domain OCT systems, Fourier-domain OCT (FD-OCT) imaging systems employ a novel wavelength-swept laser as a light source. (Figure 2b) The laser has a narrow spectral output that can be tuned electronically over a wide band of wavelengths (typically 1250–1350 nm, the same spectral band in which time-domain systems operate). As the wavelength of the laser sweeps, reflections from refractive-index discontinuities at different depths are encoded in the frequencies of the interference signals that they generate. An entire depth scan is reconstructed by Fourier transformation of the interference signals recorded during a single sweep of the laser. The bandwidth of the laser and width of its wavelength sweep determine the scan depth and axial resolution, respectively. FD-OCT systems can acquire images at line rates at least 10 times faster than time-domain systems without loss of image quality. This speed advantage results from the elimination of mechanical scanning of the reference mirror and the signal-to-noise advantages of Fourier-domain signal processing.
Fourier-domain OCT has been used for real-time, volumetric imaging of the eye,14 gastrointestinal tract,15 and coronary arteries.16 The principal advantage of Fourier-domain OCT in intracoronary imaging applications is that it permits the acquisition of longitudinal sequences of cross-sectional images with significantly faster pullback speeds than conventional time-domain OCT. Fast pullback speeds are essential for imaging long segments of arteries with minimal ischaemia, eliminating the need for proximal vessel balloon occlusion during image acquisition.
Technical specifications
Prototypes of FD-OCT systems are now undergoing evaluation in preparation for regulatory clearance for commercial sale. Table 1 compares the key specifications of conventional (time-domain) OCT systems with those of a representative first-generation Fourier-domain OCT system (LightLab Imaging, Inc, Westford, MA, USA). The main performance advantages of the Fourier-domain OCT system are its faster frame rate, higher image line density, and faster maximum pullback speed. To enable imaging of coronary arteries with large lumens (> 4 mm), the FD-OCT system also has a wider scan diameter. The penetration depth and resolution of the first generation of Fourier-domain OCT systems do not differ significantly from those of commercial time-domain OCT systems.
Designed for rapid-exchange delivery, the first generation of FD-OCT imaging catheters can be delivered over a 0.014-inch guidewire through a 6 Fr or larger guide catheter and have 2.5- 2.8 Fr crossing profiles. These catheters consist of a fibre-optic imaging core threaded through a hollow torque cable that rotates and pulls back within a transparent plastic sheath. To maintain high longitudinal resolution at fast pullback speeds, the torque cable rotates faster than a conventional IVUS catheter (100 rotations/sec versus 30 rotations/sec).
Preliminary experience with Fourier-domain OCT
Studies have been conducted to evaluate the safety and performance of prototype versions of the FD-OCT systems in both pre-clinical and clinical settings. The results of initial bench tests verify the improvements in image acquisition speed, lateral resolution, scan range, and other basic imaging specifications listed in Table 1. A key objective of early preclinical studies in an acute porcine model was to develop a reliable flush method that can be applied safely and effectively in the catheterisation laboratory. These experiments demonstrated that effective clearing of blood from the imaging field can be achieved by injecting saline, angiographic contrast media, or a mixture of contrast and saline, through the guide catheter. In preliminary studies, injection of 14 ml volume of contrast at a rate less than 4 ml/s was found to be sufficient to achieve an imaging period of 2-3 seconds consistently in all of the major coronary branches. At a pullback rate of 20 mm/s, an imaging period of 2 seconds is long enough to scan a 4-cm vessel segment. As saline and saline-contrast mixtures have lower viscosities, larger volumes and higher flow rates are required to achieve equivalent clear imaging times, especially in distal segments of highly branched arteries. The required flush volume depends on the proximity of side branches and the location of the imaging probe relative to the tip of the guide catheter. Therefore, fast rotation and pullback rates, combined with tight synchronisation of the movement of the pullback mechanism, are essential for imaging segments of arterial segments longer than a few centimetres.
The most extensive series of clinical studies of Fourier-domain OCT imaging to date began in October, 2007, at the Helios Heart Centre, Siegburg, Germany, in collaboration with LightLab Imaging. Over a period of six months, Dr. Eberhard Grube, who serves as principal investigator, and other senior cardiologists at the Centre (Drs. Buellesfeld, Mueller, and Gerkens) recorded pullback OCT images from 25 patients during coronary interventions, with an average of 2 pullback sequences acquired per patient. Figure 3 shows an example of an FD-OCT image extracted from a pullback image sequence recorded from one of the patients. The corresponding longitudinal (L)-mode image in the figure shows a profile of the vessel along its length. In this case, a 4.9-cm segment of the artery was imaged in 3.3 sec while contrast was injected through the guide catheter. The high resolution of the image is evident from the ability to distinguish the thin intimal, medial, and adventitial layers of the artery, with thicknesses less than 0.4, 0.15, and 0.2 mm, respectively.
The high line density of Fourier-domain OCT images is especially valuable for visualisation of stented arteries. Figure 4 shows examples of OCT images of stents that were obtained immediately after implantation and at long-term follow-up. In addition to visual assessment of the apposition of individual stents struts (Figure 4a), neointimal growth as thin as 20 µm (Figure 4b) and as thick as a 1 mm (Figure 4c) can be measured precisely along the length of the stent.
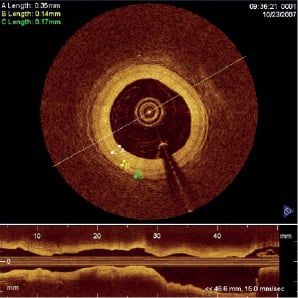
Figure 3. An example of an OCT image acquired with a prototype OCT system (LightLab Imaging, Westford, MA, USA) from the coronary artery of a patient undergoing percutaneous coronary intervention. The lengths listed in the upper left corner correspond to measurements of the thicknesses of the intima, media, and adventitia, denoted by the letters ╘A╒, ╘B╒ and ╘C╒, respectively. The L-mode image (lower quarter of figure) shows a longitudinal image of the artery acquired during a 20 mm/s pullback. [Images courtesy of Dr. E. Grube, Siegburg Heart Centre, Siegburg, Germany].
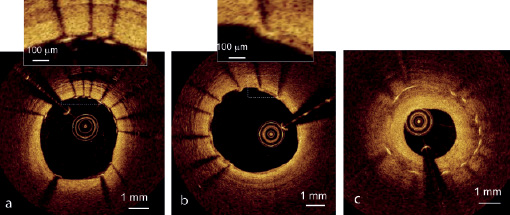
Figure 4. Examples of OCT images of stents acquired with a prototype FD-OCT system (LightLab Imaging, Westford, MA, USA). (a) Bare-metal stent imaged immediately after implantation. Stent imaged at long-term follow-up with (b) thin and (c) thick neointimal coverage. [Images courtesy of Dr. E. Grube, Siegburg Heart Center, Siegburg, Germany]
Figure 5 shows examples of Fourier-domain images of fibrous, calcified, and lipid-rich lesions acquired from target arteries of patients undergoing interventional coronary procedures. Fourier-domain images of the main classes of atherosclerotic lesions exhibit the same defining characteristics as those of time-domain OCT images described in earlier publications.2 The reduced spacing between angular samples in the Fourier-domain images improves the sharpness of plaque boundaries, which may facilitate the detection of thin-capped lesions and lesions containing small clusters of foam cells.
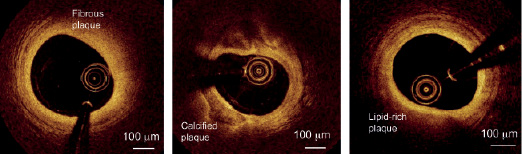
Figure 5. Fourier-domain OCT images of (a) fibrous, (b) calcified, and (c) lipid-rich lesions acquired with a prototype FD-OCT system (LightLab Imaging, Westford, MA, USA) [Images courtesy of Dr. E. Grube, Siegburg Heart Center, Siegburg, Germany].
Future developments
Continued advances in the technology of Fourier-domain OCT promise to improve the ease of use and to broaden the applications of OCT imaging in clinical practice and medical research. Quantitative plaque characterisation methods aimed at rapid identification of plaque types are under development. Computer-assisted detection of stent malapposition and denudation of endothelial coverage with OCT is also being explored. These future diagnostic tools, when combined with novel three-dimensional, colour-mapped displays, may enable cardiologists to detect therapeutic targets in real-time during percutaneous interventions.
Furthermore, novel stents including bioabsorbable magnesium stents or those made with poly-lactic acid offer an exciting era into the management of coronary artery disease with the potential to eliminate some of the problems related to permanent metallic stents. The development of such technologies, however, remains time consuming and expensive, often necessitating the sacrifice of animals to retrieve the stents.17 The high-resolution imaging capabilities of OCT gives the potential for rapid in vivo assessment of tissue response to various stent designs, thereby limiting the dependency on statistical comparisons in large numbers of animals.17 The serial use of OCT is also an attractive concept, giving unique insights into the time course of stent endothelialisation following implantation. This application has been greeted with strong enthusiasm and OCT is now being incorporated into large multicentre randomised stent trials aimed at complementing angiographic and clinical endpoints.
Conclusions
The ability to provide high-resolution imaging in vivo is one of the most significant advances attributable to OCT. With even faster pullback speeds and simplification of the procedural requirements (e.g. by eliminating the need for proximal balloon occlusion), OCT will become more accessible to a greater number of centres and operators world-wide. Such advances will also see OCT consistently applied as a multivessel diagnostic modality, able to provide a truly representative assessment of the entire coronary tree.

