Critical limb ischemia, current treatment, primary amputations, interventional revascularization, costs.
Abstract
Background: Multiple reports document the higher costs of primary amputation (PA) compared to infrainguinal bypass surgery (IBS). Recent reports document 40-50% cost-effectiveness for percutaneous transluminal angioplasty (PTA) compared to IBS. The literature suggests appropriate initial treatment for critical limb ischemia (CLI) to be IBS = 38%, PTA = 28%, and PA = 16%. The encouraging 6-month Laser Angioplasty for Critical Limb Ischemia (LACI) 93% limb salvage rate prompted an independent CLI and LACI clinical and economic analysis. Methods: Between 1999-2001 a reference amputation population (RAP) of 417 patients with at least one infrainguinal amputation were identified from a 2.5 million patients Medicare/insurance dataset. Clinical data and all medical cost claims for 18 continuous months, 12-month prior and 6-month post-amputation, were analyzed for PTA, IBS, and PA treatment pathways. Based on multiple assumptions and the LACI phase II results, economic outcomes were used for a LACI pathway analysis compared to PTA, IBS and PA pathways by substituting the LACI trial pathway as the initial treatment in lieu of the RAP actual treatment. Results: Initial treatments for CLI RAP were PA = 67%, IBS = 23%, PTA = 10%; A majority of wound complications (80%) and myocardial infarction 7/9 (77.7%), stroke 13/16 (81.2%), and death 2/2 (100%) occurred in the PA RAP. Only 35% of the RAP had an ankle brachial index (ABI) and only 16% angiography before PA. 227/417 (56%) of the RAP had multiple procedures. Average total costs / patient = $31,638 without LACI and $25,373 with LACI. Average savings/patient with LACI = $6,265. Conclusion: The most common current treatments in the US for CLI are still characterized by high rates of primary amputations, multiple procedures, and high rates of procedure-related complications. Despite the limitations and assumptions of this analysis, the utilization of a LACI pathway first revascularization treatment strategy may provide clinical and economic cost savings in treating patients with CLI.
Introduction
Critical limb ischemia (CLI) remains incompletely characterized in the clinical literature. Therefore information, knowledge, and awareness surrounding the clinical impact of CLI remains obscure. There exists an even greater paucity of data and less understanding regarding the clinical costs of treating CLI and amputation to the patient, family, and to society. It is estimated that between 220,000 - 240,000 major and minor lower extremity amputations are performed in the United States (US) and Europe yearly for CLI1-5. In the US the amputation rate has increased from 19 to 30 per 100,000 persons years over the last two decades primarily due to an increase in diabetes and advancing age6-7. Despite advances in cardiovascular treatment, in patients over 85 year of age an amputation rate of 140 per 100,000 persons/year has been reported with a primary amputation (PA) still carrying an excessively high mortality rate of 13-17%7-9. In the highest risk patients, 30-day periprocedural mortality after amputation can range from 4 - 30% and morbidity from 20 - 37%10, because many end-stage CLI patients will suffer from sepsis and progressive renal insufficiency. Successful rehabilitation in patients after below knee amputation is achieved in less than two-thirds and in less than one half after above knee amputations and overall, less than 50% of all patients requiring an amputation ever achieve full mobility11-14.
CLI: the natural history
Wolfe et al. classically described the natural history of CLI in a collation of 20 publications on 6118 patients by stratifying them into a low-risk cohort of 4089 patients (rest pain only and ankle pressure > 40mmHg) and a high-risk cohort of 2029 patients (rest pain and tissue loss with or without ankle pressure < 40mmHg)15. At 1 year, 95% of the high-risk group and 73% of the low-risk group required a major amputation without revascularization. A 75% limb salvage rate was achieved at 1 year in the high-risk group with revascularization. The cumulative probability of survival for the entire group was 74% at 1 year, 58% at 2 years, 56% at 3 years, 48% at 4 years, and 44% at 5 years. Multiple reports have repeatedly documented the poor overall prognosis for the CLI patient with mortality rates greater than 50% after three years16-17. Within one year of the diagnosis of CLI, 25% will require a major amputation and another 25% will be dead5,18.
Interestingly, recent reports by Panayiotopoulos et al. and Kalra et al. have shown significantly improved long-term survival after revascularization and limb salvage as compared to CLI patients following revascularization failure and amputation6,19. Statistically significant five-year survival rates were achieved after limb salvage in the Kalra et al. report6. Clearly the clinical costs to the CLI patient are extremely high underscoring the need for a characterization of the clinical and economic costs involved in treating CLI especially considering the incidence of CLI is expected to significantly increase yearly approaching global epidemic proportions.
CLI: the data?
Inherent problems in obtaining pertinent economic outcome information in CLI include a lack of standardization of reporting, definitions, hospital and payer charges and costs, changing technology and the lack of a consensus CLI treatment pathway between clinicians and institutes in both the US and Europe5. CLI is often treated differently by each medical specialty and treatments can vary between geographical locations. Several European CLI economic reports appeared in the late 1980’s and early 1990’s and were included in the cost analysis of the TransAtlantic Inter-Society Concensus (TASC) document reported in January, 200016. This document though did not include any of the new technologies and strategies used today in a more “modern” revascularization approach to treating CLI, limb salvage, and Primary Amputation (PA). Unfortunately, since 2000, few data have reported the clinical and economic costs of CLI further demonstrating a need for information. Furthermore, divergent reports exists in the literature regarding the economic treatment costs of infrainguinal bypass surgery (IBS) and PA with sparse data available reporting the costs of percutaneous revascularization procedures for treating CLI including percutaneous transfemoral angioplasty (PTA) or excimer laser revascularization
The purpose of this analysis was to determine the clinical characteristics of treating CLI in a more “modern” US patient population and to determine the potential to improve clinical and economic care with the use of excimer laser revascularization (Laser Angioplasty for Critical Ischemia or LACI) in the treatment of CLI. To this end, we investigated standard-of-care clinical treatment pathways of patients with CLI; examined population characteristics and actual treatment patterns of a reference population of 417 CLI patients who ultimately experienced amputations; and estimated the expected clinical impact on their care if a LACI first pathway had been used in lieu of the first PTA, IBS, or PA. During this study, we examined 18 months of medical claims prior to each patients qualifying amputation from a 2.5 million patient Medicare and insurance dataset, and identified incidence and costs associated with three clinical treatment pathways, including PTA, IBS, or PA. The LACI assumptions were based on the Laser Angioplasty for Critical Limb Ischemia (LACI) Phase II clinical outcomes.
Methods
Study population
Between 1999-2001, a Reference Amputation Population (RAP) of 417 patients with at least one infrainguinal amputation was identified from a data source of 2.5 million patients in a large Medicare and commercial insurance dataset. Clinical data elements evaluated included all patient records covering inpatient hospital care, in patient rehabilitation, skilled nursing services, hospital patient care (including ambulatory surgery), physician data, pharmacy claims and other outpatient services including podiatry and home health. The data review and analysis was conducted by Strategic Health Resources®, an independent consulting and data-mining firm, and commissioned by The Spectranetics Corporation. To qualify as part of the RAP patients had to meet all of the following criteria:
A. A lower amputation of any kind during the final six months of the 18-month study period. The final amputation during this period became the “qualifying amputation” for the purpose of establishing the study period. This resulted in a data evaluation for 18 continuous months on each RAP, 12-month period prior and 6-month post amputation.
B. Continuous insurance eligibility for 18 months prior to the date of the qualifying amputation.
C. Documented CLI based on having at least one qualifying diagnosis or specified combination or diagnosis and procedure codes in the patients record prior to or concurrently with the amputation.
To be included in the RAP at least one of the following diagnostic criteria had to be documented:
A. Documentation of lower extremity atherosclerosis with rest pain or ischemic ulceration or gangrene; or,
B. Documentation of gangrene alone, only if it occurred in conjunction with hospital records specifying amputation associated with peripheral vascular disease (PVD), or,
C. Documentation of diabetes with manifestation of PVD, if and only if it was present as the principle diagnosis for the qualifying amputation.
To avoid inadvertent inclusion of patients whose amputations could relate to non-CLI etiologies, specific exclusions included:
A. All patients with a cancer diagnosis.
B. Any “accident or injury” codes.
C. All patients with a paraplegia or quadriplegia code.
Utilizing these criteria, a RAP of 417 CLI patients was obtained with an average age of 70.9 years. The RAP was 59% male and overwhelmingly 82% diabetic.
CLI treatment pathways categories
To characterize the process of care, we defined treatment care pathways identifying common sequences of key procedures and grouped them according to the first index key procedure recommended for CLI treatment including PA, IBS, and PTA. We identified common sequences of subsequent key procedures and grouped them into nine treatment care pathways (Table 1).
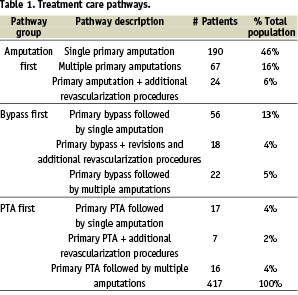
Based on our comparisons of recommended treatment of CLI to the clinical pathways identified in the study, it appears that PA was used to a much greater extent than the clinical literature suggests, while PTA and IBS procedures appear underutilized. Specifically, 67% of patients in our study population had a PA as their first index treatment, while the literature suggests this approach would be best for approximately 16% of CLI patients20-23. In contrast, 23% of patients had an IBS as their first CLI treatment, while the clinical literature suggests this approach for an estimated 38%20-23. Likewise, in the RAP, 10% of CLI patients had a PTA first treatment, while the clinical literature recommends this approach for an estimated 28%20-23.
Claims analysis
For each of the 417 CLI patients in the RAP, all claims for 18 continuous months prior to the qualifying amputation were evaluated. For patients with multiple amputations, all claims for 18 months prior to the first qualifying amputation occurring during the final six months of the study period, continuously through the last qualifying amputation were included. All claims related to procedures for the treatment of CLI were evaluated and divided into the following categories:
• Diagnostics and evaluation - PVD assessment and patient evaluation for treatment prior to or concurrent with the first key procedure - identified by best practice clinical algorithms taken from literature review.
• Pre-op care - Visits coded as pre-op exams prior to a key procedure.
• Revascularization procedures/amputations - Any amputation, IBS, or PTA (“Key Procedures”).
• Key procedure episode - Services provided during the outpatient or inpatient stay (including rehabilitation) for any key procedure, excluding dialysis-related care.
• Post-procedure care - Defined as routine post-procedure care (relevant physician visits, home health, revisions, and appropriate services) to amputated stumps, verified through discussions with clinicians.
• Procedure-related complications - Defined as all complications occurring within closely defined time periods following a relevant key procedure, or infections. Complication definitions were taken from the literature analysis and verified through discussions with clinicians.
• Pharmaceutical use - Defined as CLI-related medications taken during the study period.
Results
Clinical data analysis
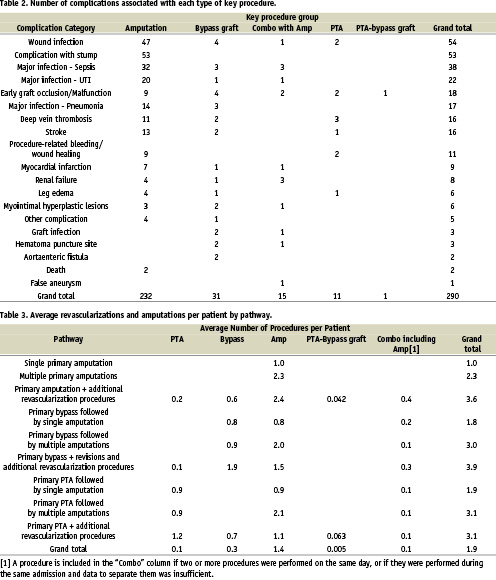
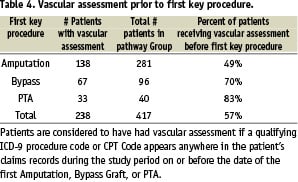
Procedure-related complications were a frequent occurrence in the RAP. Overall 290 complications were identified with 80% associated with an amputation. Wound infections and stump dehiscence were the most frequent complications and myocardial infarction, stroke and death were associated with amputations (Table 2). Multiple amputations and revascularization were also frequent in the RAP (Table 3).
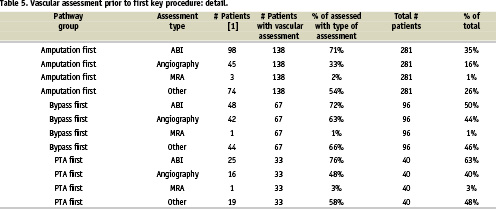
An analysis of the CLI patient noninvasive and invasive diagnostic pre-procedural work-up prior to a PA was performed. Shockingly, less than one half (49%) of the RAP had any diagnostic vascular evaluation prior to a PA with the incidence of ABI, angiography and MRA being 35%, 16%, and 1% respectively (Table 4-5).
Clinical practice analysis
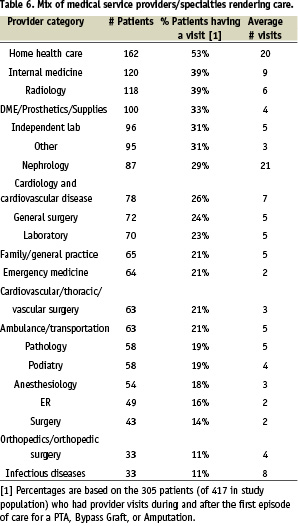
An evaluation of the physician and medical service providers / specialties seen by the RAP during and between episodes of CLI treatment was obtained (Table 6). The percentage of radiology, cardiology, and vascular surgery services provided were 39%, 26%, and 21% respectively.
LACI clinical outcomes
The Laser Angioplasty for Critical Limb Ischemia (LACI) trial was a prospective registry to evaluate limb salvage rates in poor or non-surgical candidates (patients who were likely to receive an amputation) who underwent excimer laser assisted revascularization. The LACI phase II trial enrolled 145 patients with 155 critically ischemic limbs (rest pain and/or ischemic ulceration with established tissue loss) with 423 lesions treated with excimer laser at 15 US and German sites25. Periprocedural results included no deaths or acute limb ischemia and a 96% laser/PTA success rate with 90% receiving “straight-line follow” to the foot. Results at 6 months included only a 2% requirement for IBS, 16% overall secondary reintervention rate, and 93% limb salvage rate. The LACI phase II study demonstrated that laser assisted endovascular intervention in this fragile CLI population results in excellent limb salvage rates with low complication and secondary intervention rates without adding excessive clinical risks.
CLI treatment pathways with LACI
As mentioned above, all patients treated in the LACI II trial were poor surgical candidates who would have required PA if revascularization was not performed. As such, the LACI patient population may resemble this RAP series. These 417 patients were analyzed to impute the potential outcomes if the LACI procedure were performed. Potential outcomes of the LACI procedure were defined in three pathways: LACI with total limb retention; LACI with a reintervention; and LACI, with or without a reintervention, followed by an amputation. Based on LACI Phase II results, we allocated the LACI patients into the three pathways as follows:
• LACI with total limb retention (62%)
• LACI + downstream reintervention (13%)
• LACI +/- downstream reintervention + amputation (25%)
Thus, the 65% of patients converting to LACI are expected to have the following distribution:
• 40% LACI with total limb retention
• 9% LACI + downstream reintervention
• 16% LACI +/- downstream reintervention + amputation
Accordingly, we estimate the average CLI patient costs treated with LACI in lieu of the first PTA, IBS or PA for the three LACI pathways. The cost for LACI with total limb retention was developed from the following components:
• The cost of “simple” PTA (with serious adverse events and a length of stay of four days or less);
• Additional physician and outpatient facility reimbursement under standard Medicare policy for use of the laser (we assume that commercial insurers will adopt the same differential on average);
• Normal follow-up care (for a simple PTA);
• An allowance for treatment costs of SAE in patients who did not experience post-hospital reintervention or amputation. (Post-hospital reintervention or amputation moves the patient to a different pathway);
• An allowance for treatment costs of subsequent lesions, which we identified in 6.5% of our study population. (Use of LACI in one lesion is assumed to have no impact on the development of disease in another lesion).
Economic data analysis
The total average cost of each of the nine CLI treatment pathways was calculated beginning with the first key procedure and included all services, complications, procedures and related costs.
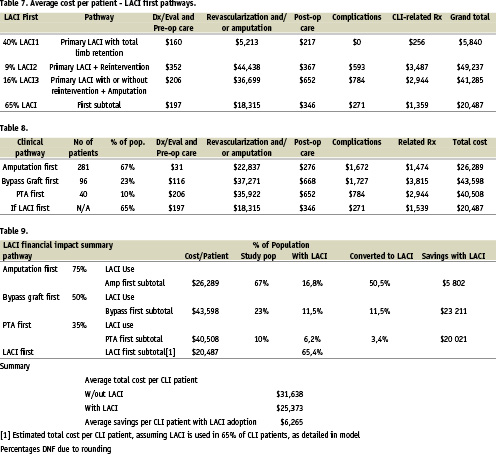
Applying the LACI assumptions and calculations, we estimated the average CLI patient treated with LACI in lieu of first PTS, IBS, or PA would generate $20,487 in medical costs for CLI-related procedures and costs over a period of six months during and after the LACI treatment. A detailed breakdown of costs by pathway was calculated for the LACI first group as compared to the standard therapies evaluated in the RAP group (Tables 7 and 8).
Across the entire RAP, the average costs per patient for CLI-related treatment was $31,638. Extrapolating the data from the LACI trial and applying it to 65% of the RAP group, it is estimated that use of LACI would result in an average cost per CLI patient of $20,487 therefore generating a savings of $6,265 per patient across the entire CLI population (Table 9).
Discussion
Clinical CLI data on the treatment of CLI suggest that almost all patients should undergo a vascular assessment and a high percent of CLI patients should be recommended revascularization to avoid amputation. Despite this noble ideal, an analysis of actual reimbursement claims data suggests that a significant majority of patients in the U.S. are still “treated” with primary amputation (PA). The clinical and economic costs of PA as a standard therapy are high, when compared to revascularization and limb salvage26-29.
In 1978, Stoney et al. proposed a PA as the best cost-effective solution to treating CLI26. However, it has never been demonstrated scientifically that a PA is a cost-effective solution in CLI. The costs of a PA reported between 1985-1994 were found to vary from $12,397 by Yin et al. who excluded rehabilitation to $40,563 ± $4,729 reported by Mackey et al. in 1985 who included rehabilitation and longer term follow-up12,27.
In 1997, Luther et al. analyzed the cost of PA in a population of institutionalized, nursing home, patients versus previously active noninstitutionalized patients28. The costs were highly variable from $13,000 in the institutionalized to $70,000 for the noninstitutionalized PA patient still living at home. The professional nursing care costs after an amputation in the US home has been estimated at $100,000 per year29. Johnson et al. attempted to characterize the costs to the patient and family of home alterations to accommodate an amputee and item ranged from $700 for a toilet seat to $25,000 for concrete wheelchair ramps30. Clearly there are indications that amputations result in a high cost to society by requiring long-term care for the amputees that cannot be rehabilitated to mobility, especially in the elderly age patient11-14,29-30.
PA is associated with high mortality and morbidity and the functionality and quantity of life is reduced for the amputee30-32. IBS and resultant limb salvage have been reported as excellent solutions for treating CLI. Reported advantages of IBS versus PA for CLI include: significant limb salvage rates, decreased 30 day mortality and morbidity, improved functional status and quality of life, cost effectiveness, and improved long term survival6,19,31,32-33. Reports by Thompson et al., Chetter et al., and Johnson et al. have consistently shown improved functional outcomes and quality of life scores in patients after limb salvage versus amputations30,34-35.
In 1992, Cheshire et al. reported that IBS, including secondary procedures, was 47% more cost effective than PA when using autologous vein and 6% more cost effective when utilizing a prosthetic conduit35. In 1997, Panayiotopoulos et al. reported PA as three times more costly that IBS and limb salvage in both diabetics and nondiabetics with costs being PA = $24,460 and IBS = $8,64036. Several other reports document the costs of successful IBS as between $16,000 and $20,00037-40. Mackey et al. reported a 2 year follow up cost for successful IBS of $20,300 if uncomplicated but quoted costs of $42,000 when secondary amputations were required13. Korn et al. reported the IBS results in CLI patients with end stage renal disease (ESRD) on dialysis and reported 67% 1-year limb salvage rate. Cost analysis was determined to be $44,308 per year of limb salvage41.
Kalra et al. reported the long-term survival after IBS (pedal bypass) in 256 CLI patients. Amputation and ESRD predicted higher mortality (p = 0.014, p = 0.0001, respectively) and overall 5-year survival rates after IBS and limb salvage were 60%6. The 5-year survival rate after an amputation was 26% therefore confirming earlier reports and documenting significantly worse long-term survival for patients suffering an amputation versus those CLI patients achieving limb salvage19.
Data has only recently been reported on nonsurgical revascularization for treating CLI, despite a greater than fivefold increase in the use of PTA42. These early reports evaluate PTA only procedures therefore a cost analysis of more “modern” CLI treatment with the use of stents, plaque excision, endopharmacotherapy, or laser (LACI) does not exist. In 1995, Hunink et al. compared the in hospital costs only for CLI patients treated with PTA or IBS37. The costs of PTA and IBS were respectively $11,353 ± $7,658 and $15,059 ± $7,313 if uncomplicated. Additional revascularization procedure increases the costs by a mean of $9,003 in both groups and any amputation or wound debridement further increased the costs by a mean of $24,766 ± $2,241. In 1998, Jansen et al. compared IBS and PTA in hospital costs in 583 patients for CLI43. The mean cost of PTA and IBS were $8,855 and $12,550 respectively for uncomplicated procedures with additional costs of $9,345 to $11,675 for nonfatal and fatal complications. In 2000, Laurilla et al. reported a 41% cost effectiveness of PTA versus IBS in 772 CLI patients44. The mean costs of PTA were $8,855 versus $16,470 for IBS. The cost of a reoperation-free year was $4,466 with PTA and $7,748 with IBS and the costs of a leg-year saved at 3 years was reported at $3,877 for PTA and $6,055 with IBS. These recent reports consistently demonstrate the cost-effectiveness of PTA versus IBS.
A comprehensive review of the clinical and economic CLI literature has lead us to several conclusions including:
• There is no evidence that a PA is an overall cost effective treatment for CLI or is more cost effective than revascularization with or without limb salvage. A PA should only be considered in the already institutionalized, immobile advanced CLI patient at high risk for IBS or PTA.
• PTA is more costs effective than IBS.
• PA, IBS, and PTA all require frequent secondary procedures and/or amputations, which are associated with added overall costs.
• There exists no consensus CLI treatment pathway.
• There remains poor understanding of the overall clinical and economic impact of CLI or amputation to the patient, the family, and to society.
• There exists a need for clinical information and education and even greater need for economic data regarding the treatment of CLI.
This independent 2.5 million US patient dataset analysis revealed several interesting clinical practice patterns. An extraordinarily high percentage, 67%, of the RAP received PA as their index or first treatment recommendation. The first treatment recommendation for IBS and PTA were 23% and 10% respectively. This RAP’s initial treatment recommendation differed drastically from a 1995 British audit in which 67% of their patients received revascularization. The index procedures in the British series consisted of 38.5% IBS and 28.5% PTA, and only 16% PA22-23. A similar report from the LEICESTER ROYAL Infirmary revealed a PA rate of only 10% with revascularization attempted in 79% of 188 CLI patients24. It remains disturbingly unclear as to the reason for these differences in clinical practice patterns between our RAP versus other published series.
Further insight into clinical practice patterns can also be obtained from a 1997 report by Hallett et al. in the Olmstead County Research Study evaluating IBS, PTA and PA between 1973 and 1992 in a defined community. Approximately 50% of the CLI patients presented with advanced Rutherford Class 4-5-6 and of those requiring amputation, 60-70% were as PA with no vascular assessment or revascularization procedure being performed therefore implying that CLI patients worldwide are treated similarly to the Olmstead County report and this RAP47.
Additional clinical practice pattern data was analyzed in our RAP regarding the CLI diagnostic work up and physician and healthcare provider consultations. An extraordinarily low percentage of CLI patients, 49% of this RAP, had any vascular assessment before a recommendation for PA. The RAP pathway had a recommendation of ABI and angiography in only 35% and 16% respectively before a first treatment recommendation for PA. This clinical practice pattern is especially disturbing when considering the excellent limb salvage results reports with pedal bypass, PTA, and LACI23,33,45. A 50% limb salvage rate has even been reported with “blind exploration” and pedal bypass in severe CLI patients without identifiable distal bypass targets during angiography50.
From the economic standpoint, this practice pattern is also disturbing when considering that the total costs of treating CLI in the US alone is estimated at between $10-20 billion per year3. It is estimated that just a 25% reduction of amputations could save $2.9-3.0 billion in US healthcare expenditures3. Further economic data supporting limb salvage include the known higher costs of amputations and related periprocedural rehabilitation as compared to IBS and PTA and limb salvage. Additionally, the annual cost of follow-up or long-term care and treatment for a patient has been estimated at approximately $49,000 after an amputation and $600 after limb salvage after PTA or IBS3,16,48-49.
Study limitations
• The 18-month study period represents a retrospective cross-section of time. The analysis did not prospectively collect data on the procedures included in the RAP or LACI patient populations.
• Procedure coding is not lesion or limb specific.
• Rates and costs of complications in the RAP may be understated because inpatient records typically contain many diagnosis codes and the first few codes are normally devoted to the underlying condition and major comorbidities.
• Procedure and CPT codes are specific to problems with an amputated stump, it was possible to identify amputation-related complications more thoroughly than PTA or IBS complications.
• The RAP analysis did not include CLI patients who did not receive an amputation.
• The RAP and LACI trial patients were both highly selected and different groups, not truly comparable groups, therefore obviating any definitive conclusions.
• There were significant assumptions made regarding the LACI phase II trial and their applicability to this RAP therefore conclusions based on these calculations are subject to bias.
Conclusion
In conclusion, the clinical and economic costs and consequences of CLI and amputations are both staggering and unappreciated and it is likely that CLI is approaching global epidemic proportions. Strong clinical and economic data currently exists supporting an aggressive approach for revascularization and limb salvage in almost every patient with CLI. A reasonable assumption for this study’s disturbing clinical practice pattern favoring a PA versus a revascularization first pathway is that many CLI patients are seen first, or referred first, to clinicians who cannot provide revascularization and therefore provide a pathway for amputation. As is true in most global healthcare epidemics, if a positive impact is to be made then it must start with information and education and progress to global commitments to enhance awareness and provide clinical and economic cost effective treatment. Despite the stated limitations, assumptions and potential biases of this analysis, the utilization of a LACI pathway first treatment strategy may provide clinical benefits and economic cost savings in treating patients with CLI.
Acknowledgments
The authors would like to thank Mrs. Kelly Tilbe for her technical help with manuscript preparation.

