Setting the stage
It is very difficult for coronary interventionalists in 2005 to quite understand the challenges faced by practitioners in 1985 when coronary stenting was born. Andreas Gruentzig had taught his disciples in Europe, North and South America how to treat ideal short, discrete and non calcified single vessel disease. Despite a 5% to 10% risk of emergent CABG, initial results were satisfactory and 5 year outcomes were being reported. Many groups, including the Gruentzig’s team at Emory, were exploring the path into more complex, diffuse, calcified tortuous and bifurcating disease and multivessel disease. At Emory, the EAST Study was being initiated and the BARI Trial was in the early planning stages.
It was becoming very clear to all involved, however, that advances into more complex diseases came at the high cost of an increasing incidence of abrupt closure of the artery either during the procedure or within 48 hours. Early papers focused on discerning between mechanical and/or thrombotic causes of vessel closure and Steve Ellis and I set about defining the class ABCD lesion morphology score that is still used today.
Imagine an environment where most if not all cardiothoracic surgeons were at best paranoid about the progress of PTCA and at worst openly hostile about loss of status and clinical volume to interventional cardiologists. Imagine an environment where cases could only be scheduled if there was an OR and surgeon available to “back you up” where cardiothoracic surgeons could “veto” PTCA attempts on any patient, and imagine the professional challenge and hazards knowing that depending on your patient selection and technical skills, 1 in 10 caths could end up as an emergency bypass, myocardial infarction or death.
By 1985 we had overcome most acute thrombosis after PTCA by use of large doses of aspirin, Persantine and I/V Dextran infusions. Intimal dissection after PTCA became the number one enemy, and all manner of attempts were directed to solving the problem of vessel dissection. Understand that balloon profiles were still horrendous, and most ruptured at around 10 atmospheres. Much work was directed at understanding the value of higher pressures, bigger balloon diameters and longer inflation times. Richard Stack “stepped up to the plate” with the perfusion balloon to facilitate ultra long inflations and others directed these endeavors towards lasers, rotablator and atherectomy to overcome the problems of coronary dissection after balloon dilatation.
A stent to make PTCA safe
At Emory University in 1985, Andreas Gruentzig introduced me to Cesare Gianturco, a retired interventional radiologist that had already made his mark in designing the first vena caval filter and embolization coils. Cesare had been implanting a variety of balloon expandable coil and self expanding Z stents in animals since 1980.
In 1985, Charles Dotter and Andy Cragg’s early animal work with arterial stenting was well recognized, but Palmaz’s early work and Hans Wallsten’s engineering of the Wallstent was still well under the radar. It took many frustrating months of work in the animal laboratory to conclude that the precise placement of the self expanding stent in the coronary arteries was difficult and unlikely to have immediate clinical application. It is of interest to note that now, 20 years later, self expanding stents still have little application in the coronary arteries (Some things we do get right the first time?).
Andreas Gruentzig’s tragic and untimely death late in 1985 disrupted progress on the stent work, but as we regrouped at Emory there was a strong feeling of purpose in pursuing Andreas’s dream of a safe, effective and widely applicable percutaneous revascularization method for the coronary arteries. In the fall of 1985, we began work in earnest with the Cook, Inc. balloon expandable Gianturco coil stent.
Step back for a moment and consider the challenges!
If anyone should have concerns for fractures of stents in the superficial femoral artery today?
Consider for a moment the concern and paranoia surrounding the placement of metal stents in the constantly twisting and bending coronary arteries; 80 times a minute –millions in a lifetime! The specter of metal shattering into the pericardium, myocardium and chest cavity was widely discussed. Our cardiothoracic colleagues, hearing us surmise about a safer and more “independent” PTCA procedure were angered even further, and the application of coronary stenting, as the next months would show, was to add “insult to injury.”
Before studying the medium term outcomes in the dog and pig coronary model, we had to get the stent in place. Let us back up again for a minute - balloons were stiff primitive 4F devices as were guides, let alone find one to fit a dog coronary artery –and animal lab x-ray facilities were primitive in the extreme. We soldiered on under difficult conditions testing different metals– (gold, tantalum, and titanium, stainless steel, gold & copper), different wire diameters and wrap configurations. Joe Brown R.T. and Keith Robertson PHD were stalwarts in the trenches during this work.
In these early days, we defined the characteristics of the ideal coronary stent including ease of placement, visualization, precise positioning, reliable expansion, biocompatibility and freedom from late mechanical failure. All are still in play as we continue to improve the technology today.
Eventually we got consistently good results with a stainless steel wrap and angiograms and histology at 6 months were flawless. Still unwilling to submit to my own prejudices, I sent the histology to Bill Roberts at that time at the N.I.H. for his independent histopathology review. I waited anxiously for over a month, but he finally called to say he could see no fractures, no adverse reactions at all to the chronic placement of this metal in the coronary artery. It was one small step for coronary intervention, but we still had no data in atherosclerotic arteries or good electron microscopy on how the metal was covered by endothelium and the important time course for these events.
We pursued a great deal of basic science with the support of Brian Bates from Cook, Inc. The “tipping point” occurred in late 1986 when doing stenting work on severely atherosclerotic rabbit iliac arteries. Balloon dilatation in one rabbit caused a severe semi-occlusive linear dissection. Placement of a 3 mm stent produced an excellent angiographic result and at sacrifice 6 weeks later, the dissection had healed completely with excellent endothelialization of the stent.
By 1987, 100,000’s of patients were being treated with balloon angioplasty with 10,000’s going to emergency surgery with a myocardial infarction rate of 50%, and in some series 10% mortality. It seemed to me that we had to employ stenting at least as a “bail-out” for failed PTCA.
I put together an investigator sponsored I.D.E. in 1987 and submitted all our preclinical data to the FDA. The medical device companies were “gun shy” about implant device failure after the recent Bjork-Shiley Value issues in the United States and there was only tacit support from nervous cardiology colleagues at Emory. Cardiothoracic surgeons and the powerful CT anesthesia lobby were outright hostile that we would “monkey” around with stents to repair our “bad outcomes” and deny them the ongoing opportunity to “control” and ridicule our PTCA work. Without Andreas Gruentzig’s powerful leadership it was a lonely battle and it was to get worse.
The Emory IRB approved the pilot study of 15 patients but insisted that all patients had to proceed to elective surgery someday. I acquiesced.
The first patient had an abrupt closure of a dominant RCA on the table. (Figure 1a). It was September, 1987. A 3 mm x 20 mm Gianturco-Roubin stent was placed across the occlusive dissection (Figure 1b).
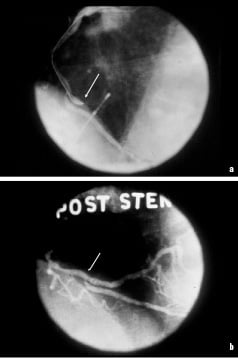
Figure 1. a. Abrupt closure of a dominant RCA in 1st stent patient, Sept. 1987. b. Same patient after placement of the of the Gianturco-Roubin stent.
The rugged truck driver had immediate restoration of flow, resolution of chest pain and EKG changes. He had an uneventful post operative course and with the hindsight of history totally unnecessary CABG but did very well. The stent remained patent at 6 month follow-up.
A dozen or more patients were similarly treated before we secured the green light to perform “bail-out” stenting without sending the patients for CABG.
Liberation
So began a new era in the history of coronary intervention. But all was not well at the “Emory Ranch”. The coronary surgery faction continued to be “stung” by the liberation of the interventional cardiologists. After presenting the first series of coronary stent patients to a packed room at the AHA in 1987, I was summarily suspended from clinical privileges at Emory by the new Chief of Medicine who had just taken over from the venerable Willis Hurst. Hurst, familiar with the local PTCA “wars” would never have succumbed to the ludicrous charges of research fraud that emanated mysteriously from within the cardiothoracic realm.
A rigorous investigation found the results of my work on stenting were completely accurate and from a brief period of abject despair evolved another 20 years of exciting work in cardiovascular intervention. Fully exonerated by the subsequent success of stenting in today’s clinical practice, I am now happy to relate the story and the risks young investigators take when they get too far ahead of the “cavalry charge.”
I may not have survived this year without the support of colleagues in the field. In a letter I have from Julio Palmaz in 1989, he offers his support; and congratulates me on all the basic science I had published, “without which we will not forward progress of this technique.” Cook, Inc. took over the IDE after the committee hearing in 1988 and funded the series of prospective rigorous multicentre studies that brought about commercial release of the first FDA approved stent in 1994.
Still, it took almost 7 years from the time of first implantation in man to obtain FDA approval. By 1984, it was perfectly clear to all that the stent had remarkable potential to improve the safety of PTCA, but some 12 months after submission of the “bail-out” data, the approval letter sat in a stack of papers on a desk in Washington, DC. Meanwhile thousands of patients were having emergency CABG with attendant morbidity and mortality.
The story surrounding final approval is worth relating. Early in 1994, Gerry Pohost, Chief of Cardiology at the University of Alabama, Birmingham received a call to say the senior senator from Alabama was in trouble at Walter Reade Army Hospital in Washington and needed a stent. Gerry Pohost called me at home to say he was “air vaccing” the Senator, the late Howell Heflin to Alabama for a bail-out stent. The senator had undergone PTCA of a distal RCA, had a severely dissected vessel and was having ongoing chest pain and EKG changes.
Heflin arrived at UAB, was promptly stented and discharged well 5 days later. Two weeks on, he gave a speech in the United States Senate extolling the virtue of the stent and behold 2 weeks later, Cook Inc. received the approval letter!
Early challenges in clinical application
This began a huge effort in educating thousands of interventional cardiologists in the use of the stent. Although some of us had been using the device for almost 7 years, the device had been severely restricted to a few investigational studies. A small cadre of us including Larry Dean, the late Cass Pinkerton and Barry George criss-crossed the United States to packed halls of up to 500 cardiologists eager to learn how to use the stent. This was the first and maybe the only “must have on the shelf” device in the history of interventional cardiology.
Recall this era with high profile, difficult to track devices. We needed to use 8F guides (sometimes even 9F), stiff 0.018” wires and advanced guiding catheter and guidewire skills to get these primitive devices in place. The early Cook-Gianturco Roubin stents were 20mm in length and at that time we did not appreciate the huge advantage of making and deploying a series of shorter, more trackable stents to get the problem solved.
Today, as I use the remarkably trackable stents now available (through 6F or 5F guides) I marvel that we had the success we achieved. Even the shorter Palmaz-Schatz stent was not any better since it was less flexible and required a retractable sheath to keep in place on the balloon.
Then there was the issue of sub-acute thrombosis in the era before ticlopidine, clopidogrel and intravenous antiplatelet agents. And, with prolonged use of heparin, coumadin plus aspirin and persantine came the inevitable femoral bleeding complications.
In 1986 Cook Inc. began investigations with a much lower profile GRII stent (Figure 2).
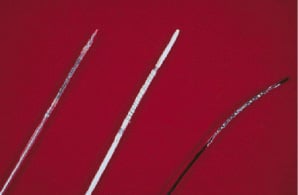
Figure 2. The 1986 model GRII Cook, Inc. lower profile stent
This markedly improved placement success. The device was the first polymer coated stent. This was an effort to reduce the risk of thrombosis but may have also contributed to an increased risk of restenosis within the stent. The biggest mistake was again to only produce the stents in 20mm and 40mm lengths as it was Cook Inc.’s sense that this would make the stent more economically viable in the market place. A randomized head-to-head comparison with the then approved Palmaz-Schatz stent compared a 20mm and a 40mm Cook stent with a 15mm Johnson and Johnson P.S. stent. The outcomes that showed superiority of the P.S. stent took the G.R stents off the market. Knowing now what we know about bare metal stent length and restenosis this was a flawed trial. Interestingly, the 35% restenosis rates seen in the long GRII stents is not different from the restenosis rates reported in the bare metal stents in the recent Johnson & Johnson Sirolimus Stent Trials. Although we were all focused on increasing the basic safety of PTCA, by 1995 we had reported the pivotal work that supported the use of coronary stents in reducing restenosis by limiting late lumen loss from recoil.
The current application of drug eluting stents has again revolutionized the field of coronary intervention. Another footnote to this fascinating era in the development of coronary stenting is the early work done with drug eluting stents. As early as 1989, my group at UAB was working on coating stents with antimitotic agents. In animal studies presented at the AHA in 1990, we presented outcomes in the pig model with methotrexate and other agents-wrong drugs, wrong time!
In retrospect those of us who met the challenges of the day can claim with pride that we provided the “proof in principle” that is fundamental to all advances in medicine. The modern stents we use routinely in today’s clinical practice may not have arrived as soon as they did without the struggles and hard work of the first 10 years.

