Abstract
Background: A 62-year old man with progressive deterioration of stable angina presented with an unstable episode and was referred for diagnostic coronary angiography.
Investigations: Physical examination, electrocardiography, myocardial perfusion scan, coronary angiography, cardiac CT.
Diagnosis: Left coronary main stem to pulmonary artery fistula.
Management: Coronary angiography, percutaneous coronary intervention, coil embolisation.
How should I treat?
Presentation of the case
A 62 year old gentleman presented with a two year history of exertional angina culminating in hospitalisation with an unstable episode. His chest discomfort came on if activity was started suddenly and strenuously, but not if he was able to warm up more gradually. At the time of admission, clinical examination was unremarkable and his ECG and CXR demonstrated no significant abnormality. Past medical history was unremarkable and cardiac risk factors were notable for a strong family history of premature coronary disease, hypertension, hypercholesterolaemia and being an ex-smoker. Two years prior to his current admission, an exercise ECG was terminated in Stage 2 of the Bruce protocol due to an abrupt increase in heart rate, but without symptoms and ECG evidence of ischaemia. At that time, echocardiography showed global left ventricular hypertrophy with preserved systolic function.
Coronary angiography revealed minor atheroma, but no obstructive lesion, in the major epicardial vessels. A fistula arose from the proximal LMS and assumed an anterior and inferior course, terminating in the right pulmonary artery (PA) (Figure 1a) (Video 1). This was confirmed by volume-rendered CT imaging and 3-dimensional reconstruction (Figure 1b,c). Myoperfusion scanning demonstrated no regional ischaemia. The fistula was deemed to be the cause of a coronary ‘steal’ and resultant angina.
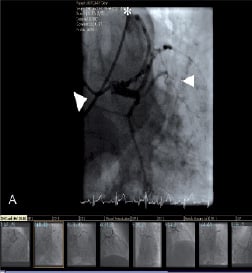
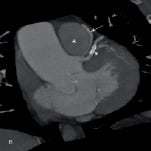
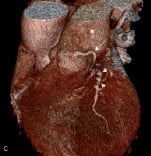
Figure 1. Pre-embolisation coronary angiogram demonstrating the fistula (arrows) from the LMS (arrowheads) to the main pulmonary artery (asterix) on cardiac catheterisation (a), oblique coronal multi-planar reconstructions (b). 3D volume rendered (CT-VR) image (c).
How would I treat?
Coronary steal with unstable angina secondary to a coronary artery fistula
The patient is a 62 year old man with stable angina in whom diagnostic coronary angiography was performed because of an unstable episode. There was a strong family history of premature coronary disease, hypertension, hypercholesterolaemia and he was an ex-smoker. Interestingly two years previously he had had an exercise ECG in which an abrupt increase in heart rate occurred without any evidence of ischaemia. Coronary angiography revealed minor atheroma but no coronary arterial stenoses. However, there was a coronary arterial fistula arising from the left main stem and draining to either the distal main pulmonary artery. Myoperfusion scanning demonstrated no regional ischaemia.
There are several questions that arise. Is the coronary arterial fistula an incidental finding and unrelated to the symptoms of angina? Is the fistula producing a coronary arterial ‘steal’ phenomenon giving rise to the symptoms of angina? Should the fistula be closed at all and if yes, should it be closed by surgery or by catheter techniques? If by catheter techniques, how should it be closed.
Coronary arterial fistulas are a rare defect but they are the most common congenital coronary arterial defects. In one study, in adults, coronary arterial fistulas have been found in 0.1% of over 33,000 patients who had coronary angiography, many of which drain into the pulmonary artery and may be an incidental finding1. Nevertheless, coronary arterial ‘steal’ has been reported to occur even in small fistulas2. A slightly worrying fact in this case is the lack of ischaemia on myoperfusion scanning so coronary ‘steal’ is by no means confirmed. These fistulas may become symptomatic if coronary arterial disease develops distal to the origin of the fistula. Such fistulas have been closed by catheter techniques and may result in abolition of the anginal symptoms despite the absence of objective evidence of myocardial ischaemia3.
Coronary artery to pulmonary artery fistulas may be remnants of a vascular connection, which was probably normal in the mediastinal mesoderm4. These may represent an anomalous origin of a supernumerary coronary artery arising from a coronary bud in the posterior pulmonary portion of the truncus arteriosus. Persistence of the connections between arterial and venous embryologic communications into adult life probably results in these fistulas. The usual course of such left coronary artery to pulmonary arterial fistulas is superior to the anterolateral part of the base of the pulmonary artery, and circles around the posterior part of the pulmonary artery before forming a connection with it5.
In the absence of any significant obstructive coronary arterial disease, there is no indication to close the fistula by cardiac surgery. Therefore, when a decision is made to close such a fistula, a catheter technique is the method of choice. So, how should this fistula be closed? A slightly confounding factor in this case is the point of origin of the fisula – the left main stem. If it had arisen from a branch distally, a covered stent could have been implanted to cover the origin of the fistula. In this patient, the left coronary artery should be engaged with a Judkins left coronary guiding catheter. Through this, a Tracker-18 or Micro-Ferret (3 Fr) catheter can be passed over a 0.014” exchange length coronary guidewire. The floppy guidewire is manipulated into the fistula vessel and passed right up to the stenotic point of entry into the pulmonary artery. The 3 Fr catheter is gradually and carefully passed over the wire up to the stenotic point. After check angiography through the guiding catheter and measurement of the fistula vessel, coils are used to close this fistula. The choice of coils is between the Interlocking Detachable Coil (IDC) or the Detachable Coil System. These are controlled-release coils, which are fully retrievable up to the point of release. These have a 0.018” calibre and can easily be passed through the Tracker or Micro-Ferret catheter. The initial coil diameter is important as it must stay in place and not pass through the fistula and embolize inadvertently into the pulmonary artery. Generally a coil which is approximately 20-30% larger than the fistulous vessel is chosen. After deploying the first coil, further coils can be implanted in order to form a tight pack, as only then complete occlusion is likely to be achieved. Furthermore, these coils are made of platinum, are non-fibred and so take a long time to thrombose and therefore it is better to arrest the flow by the end of the procedure with the tight pack of coils. Also, at the end of the procedure, selective coronary angiography should be repeated to ensure that there are no other smaller fistulous vessels apparent either in the left or in the right coronary artery, which may cause problems in the future. For successful closure of this and any other coronary arterial fistula, a variety of specialised equipment will be needed in the catheterisation laboratory6-8.
The catheter intervention procedure in such a case is relatively easy with very small chance of complications such as inadvertent embolisation of the coil and myocardial infarction. If a coil were to embolize, it would do so to the pulmonary arterial branches, from where it can be retrieved8. The incidence of myocardial infarction in this fistula is very low. Nevertheless, the patient will not come to any harm with the use of prophylactic aspirin indefinitely.
How could I treat?
The Invited Experts’ opinion
Congenital coronary arteriovenous fistula (CCAF) is a rare anomaly, mostly recognised on echocardiography or on selective coronary angiography and not uncommonly without symptoms1-3. Most often, the coronary arteries arise normally from the aorta but show a fistulous communication usually with a cardiac chamber. This fistulous connection may be of variable size and can be found in any part of the course of the coronary artery of which the segment proximal from the fistula may be enlarged. Most CCAF can be found on the right coronary artery and most of the CCAF drain into the coronary sinus, right atrium or right ventricle3. The pathophysiology of a CCAF depends on the site of drainage of the fistula and the volume and duration of the abnormal flow2,3. In this present case the fistula runs from the proximal left coronary artery and drains without obstruction into the pulmonary artery.
Treatment of a coronary arteriovenous fistula should be based on clinical symptoms and exact anatomic diagnosis2. Until recently, coronary angiography was the gold standard in this regard, but CT scanning or MR imaging with 3-D analysis is rapidly developing into an additional important diagnostic mode. 3-D analysis has the crucial feature of allowing the representation of the interrelationship with relevant cardiac structures. In the present case, indeed coronary steal may result in inadequate blood flow to the distal left coronary arterial branches, resulting in anginal complaints. 3-D CT scanning nicely demonstrates the local anatomy of the CCAF in relation to the aortic and pulmonary root.
Adequate treatment of a coronary arterial fistula should result in closure of the fistula with preservation of the normal coronary arterial bed without intervening with other cardiac structures2. In doing so, no vascular remnants should be left behind that may predispose to local and coronary thrombosis or to endocarditis. How the CCAF should be closed depends on the anatomy of the fistula. If closure is amenable for catheter technique, this would be the first option3. In this case, catheter delivered closure material can be anchored in the fistula without much risk of dislodgement or embolisation. Should this result in complications (either with regard to the coronary arterial bed or to the surrounding structures like the pulmonary root) or failure of closure, than I would be most happy to supply definite closure of this CCAF, considering off-pump surgery and contemplating a minimally invasive approach. The procedure would include closing the fistula both at its proximal and distal end.
How did I treat?
Actual treatment and management of the case
It was felt that surgical access to the fistula and ligation would be difficult, so closure of the fistula by percutaneous embolisation was proposed. Femoral arterial access was used for the procedure and a 6 Fr Extra Back-Up (EBU) 4.0 guiding catheter used to selectively engage the left main stem. The fistula was entered with a Venture (Velocimed, Minneapolis, USA) directable catheter (0.017”) and a 300 cm PT2 guidewire (0.014”). This was followed by the deployment of a controlled release 6 x 12 mm 3D Nexus nitinol coil (EV3), more typically used in neuro-interventional procedures, within the fistula, using an Echelon micro-catheter. Fistula flow was finally occluded after the application of 0.4 ml Onyx, a radio-opaque injectable liquid non-adhesive polymer which solidified over the coil (Figure 2) (Video 2). Significant traction was necessary to remove the distal catheter tip from the Onyx, leading to severe bradycardia and the need for temporary venous pacing. Spasm of the left anterior descending artery also occurred during removal of the catheter, requiring intracoronary nitrates to settle. The pacing wire was removed after some minutes and the patient was transferred in a stable condition to the ward. He was discharged the following day without further complication and advised to take aspirin 75 mg once daily and clopidogrel 75 mg once daily for one month. Multi-slice CT and reconstructive CT scanning after 3 months confirmed persistent closure of the fistula (Figure 3). At nine months post procedure, he remained free of limiting angina and less fatigued.
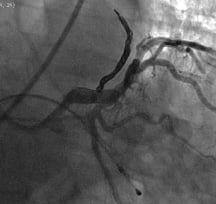
Figure 2. Post embolisation with coil deployment and onyx cement shows angiographic closure of the fistula.
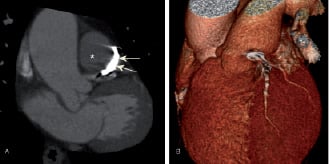
Figure 3. Repeat oblique coronal multi-planar reconstruction (a) 3D CT-VR imaging (b) Three months post percutaneous coil embolisation shows coil in-situ with persistent closure of the fistula.
Discussion of diagnosis
Incidence & clinical features in adults
Coronary artery fistulas are rare congenital malformations defined as direct vascular connections from a coronary artery to a cardiac chamber or major mediastinal blood vessel without an intervening capillary bed. The true incidence of coronary artery fistulas is speculative since many may be small and never detected, or only detected incidentally with imaging for another indication. At least 75% of coronary artery fistulas found incidentally are small and clinically silent, but as in the case presented can provoke coronary ‘steal’ manifesting in angina1. Other recognised complications include thrombosis and embolism, cardiac failure, atrial fibrillation, rupture causing haemopericardium, endocarditis/endarteritis and arrhythmias2-4. Larger fistulae result in shunts and the resultant haemodynamic consequences lead to volume loading in the drainage chamber(s), giving rise to right ventricular and/or bi-ventricular failure in the longer term if left untreated. Resolution of symptoms have been reported with percutaneous or surgical closure of the fistula5. Coronary artery fistulas can originate from almost anywhere in the coronary vasculature and terminate in any of the cardiac chambers, great veins, or pulmonary arteries. There may be multiple feeding arteries to a single coronary arterial fistula drainage point, or there may be multiple drainage sites. Small, asymptomatic fistulas arise much more commonly from the left coronary system6. In nearly all series, over 90% of fistulas drain to the right side of the circulation. Drainage to the left ventricle is least common4.
Surgical closure of coronary artery fistulas
Surgical closure of coronary fistulae, by external placation on the beating heart or by intracardiac closure using cardiopulmonary bypass, is the historical gold standard mode of treatment5. It is indicated in patients who require operative repair of other complex (and invariably congenital) cardiovascular pathology and can also allow for reduction in the size of very large aneurysmal dilations of either the fistula or the proximal coronary artery6. Approximately 10-30% of patients with a coronary artery fistula also have another congenital cardiovascular anomaly7, most commonly Tetralogy of Fallot, patent ductus arteriosus, and atrial septal defect. Mortality related to surgical closure of isolated coronary artery fistulas has been reported to be <1%8 but the risk of recurrence or residual shunts is well recognised in longer term follow-up7. Recurrence may be due to the presence of multiple fistulas which may be unmasked only once the larger fistulas, appreciated by imaging, have been surgically closed.
Catheter device closure of coronary artery fistulas
Transcatheter closure of coronary fistulas is also now an accepted effective and safe alternative to surgery in adults8,9. The principle of catheter based techniques is to advance a delivery catheter to the most distal segment of the fistula (beyond any branches that may feed normal myocardium) and deploy an occluding device in that location. Such a strategy was initially reported in 1983, using a detachable occlusive balloon10. Several institutional case series have since reported favourable outcome data with an expected mortality rate of <1%. Periprocedural complications including ST changes, myocardial infarction11, arrhythmias and fistula dissection have been reported. Occluder device embolisation is also a recognised complication which occurs as a result of high velocity flow in the larger fistulas or with under-sized coils12. Embolised coils can be retrieved with snares.
Many different types of occluding devices have been utilised including detachable balloons, Gianturco and other types of coils, Rashkind and Amplatzer PDA and ASD closure devices, Grifka Bags, and Amplatzer Muscular VSD closure Device, and Vascular Plug and has recently been comprehensively reviewed1. Deployment of a covered stent in the main coronary vessel, over the origin of the fistula, has also been described as a successful approach13 and maybe particularly useful if there is co-existing coronary atheroma.
A percutaneous strategy to occlude fistulas may not be feasible in a minority of patients due to excessive tortuosity, small lumen calibre, the presence of multiple drainage sites or the presence of normal coronary branches too close to the drainage site to allow selective occlusion. Indeed, small residual or recurrent leaks have been seen in approximately 10% of patients treated by catheter techniques8. For small fistulas, 3 Fr micro-catheters co-axially positioned within a larger coronary guiding catheter, could be used for coil deployment. In the case presented, the acute angled origin of the fistula arising from the proximal LMS, coupled with the more distal tortuosity, repeatedly resulted in prolapse of the guidewires into the proximal LAD. This necessitated the use of a steerable Venture catheter to provide the additional support and facilitate access of further kit into the fistula and its closure. This is the first case report, to our knowledge that describes the use of a steerable coronary catheter, for use in this setting.
Long term outcome
Little data exists regarding the long term outlook for adult patients with coronary artery fistulas following surgical or transcatheter closure. Cheung et al reported on the follow-up of 41 asymptomatic, mostly adult, patients after surgical fistula closure7. Coronary angiograms were performed on 21 patients and a significant persisting abnormality was confirmed in 20 of these individuals, such as native proximal coronary dilatation or atresia and thrombosis of the distal native coronary vessel. Four patients had demonstrable fistula recurrence, at the exit of the original fistula or at a previously unseen exit location, but without haemodynamic disturbance.
Most literature on long term follow-up is derived from the paediatric population, where transcatheter occlusion of congenital coronary fistulae is the preferred means of treatment in experienced centres. Persistent native coronary artery dilatation has been documented4 years post closure of large fistulae in four children aged 1.5-3 years old14. As with adults, residual fistulae are also observed, despite the absence of symptoms15. Follow-up studies one to 31 months (mean, 14.6 months) after coronary fistulae occlusion in 13 children noted only four patients with trivial (clinically insignificant) residual shunts in an experienced Canadian centre16.
Thus, even though most patients are asymptomatic after coronary artery fistula closure, they should not be dismissed from follow-up. The paucity of data after closure of coronary fistulae in adults is, arguably, sufficient justification for long term monitoring. It may be prudent to follow them with appropriate tests to evaluate myocardial perfusion. Long term anticoagulation with at least an antiplatelet agent appears to be advisable; the role of warfarin therapy in the presence of persisting aneurysmal dilation of the parent coronary arteries is currently unclear. The type and duration of subacute bacterial endocarditis prophylaxis after complete fistula occlusion, by coil embolisation, is another area where guidance is lacking.
Conclusion
Coronary artery fistulae are a relatively rare entity, but they may give rise to such complications as ischemia, cardiac failure, thrombosis, arrhythmia, endocarditis, and even rupture. Catheter interventions for residual small shunts after either surgical or catheter interventions are readily repeatable if necessary. Because of a much shorter recovery time and avoidance of a scar, transcatheter closure is considered the procedure of choice in applicable patients.
There appears to be good consensus that all symptomatic patients should undergo closure of medium or large coronary artery fistulae. However, the decision of whether, and when, to submit a patient with a clinically detectable, but asymptomatic moderate or large fistula to a procedure is more difficult. A conclusive trial of intervention versus novel therapy or expectant medical management for these patients with the worst coronary artery fistulas will be nearly impossible because of the relative rarity of this specific anatomy.
Sometimes, the absence of objective functional evidence that the fistula is the culprit cause of the patient’s symptoms, can further complicate the clinical decision-making and timing of any planned intervention, as was the case in our patient. Whether trivial to small (not clinically discernible), asymptomatic fistulae require closure simply because they are detected incidentally is debatable. In such patients, the broader clinical context needs to be considered, such as the patient’s latent risk for endocarditis or if they are undergoing an invasive procedure for another cardiac problem.
Online data supplement

