Abstract
Coronary artery perforation is a serious complication of percutaneous coronary intervention (PCI). This review summarises the aetiology, incidence, current existing therapeutic options and outcome of coronary perforations given the advance in interventional techniques, devices and use of glycoprotein inhibitors (GP IIb/IIIa). Perforations are classified as Type 1, 2, or 3, as previously defined. Type 1 and 2 perforations are predominately caused by hydrophilic and stiff wires and do not require pericardial drainage or surgical intervention. Type 3 perforations are more often associated with stent and device use and can be initially managed by percutaneous methods.
Introduction
Although interventional cardiology had its beginnings in the late 1970s, it still remains a vigorous discipline with continued rapid evolution. The number of percutaneous coronary interventions (PCIs) performed worldwide is increasing. With the improvement of technology and interventional tools, the number of procedures is expected to increase further. There are still multiple challenges to be met with, and creative solutions to be found. With the increasing armamentarium available to the interventional cardiologist, lesions, which were earlier under surgical preserve, will no longer be so. These increasing challenges however will beget more complications.
Among the most dreadful complications seen during PCI are abrupt vessel closures and perforations. The improvement in stent technology and in antithrombotic therapy has resolved almost completely the need for emergent surgical intervention for cases of abrupt vessel closure1. In contrast, as calcified, tortuous, and occluded vessels are increasingly treated by percutaneous means, vessel perforation is likely to persist. In addition, stiffer guidewires and high-pressure post-stent balloon dilatation, may further negatively influence the clinical consequences of perforations. The widespread use of glycoprotein (GP) IIb/IIIa inhibitors, may worsen the consequences.
This review summarised the available information regarding coronary perforations and details the current existing therapeutic options.
Incidence
The incidence of this rare but potentially life threatening complication is low following balloon angioplasty and seems to occur more frequently with the use of more aggressive coronary interventional devices. Angiographic evidence for perforation has been reported in 0.1% of lesions treated with balloon angioplasty (PTCA) and 0.5%-3.0% of lesions treated with high-speed rotational atherectomy (0.8%), directional coronary atherectomy (DCA) (0.9%), laser (1.0%) and stents (0.2%)2-6.The incidence of this complication along with their outcomes in various studies has been summarised in Table 17-15.
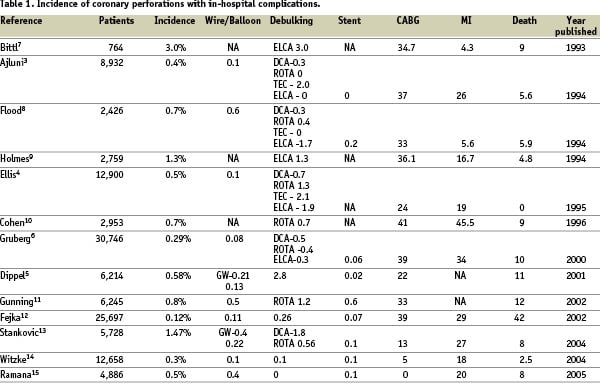
In a study by Seshadri et al16, perforations accounted for 20% of referrals for emergency bypass surgery. They also reported that coronary perforation occurred more frequently with debulking techniques (rotational and DCA) than with non-debulking (PTCA and stent) techniques (1% versus 0.26%; p < 0.001).
Perforations are also more likely to occur when intravascular ultrasound (IVUS) is used. The main reason is an incorrect interpretation of the information provided by IVUS and the usage of compliant oversized balloons to maximise the stent lumen area13,14. Furthermore, its magnitude and management may be further complicated with the use of GP IIb/IIIa platelet inhibitors.
Predisposing clinical and angiographic characteristics
Perforations constitute part of the price one pays for an increased success rate. It occurs when a dissection or intimal tear propagates outward sufficient to completely penetrate the arterial wall. Advanced patient age and female gender seems to predispose to this complication. Females are probably more prone to this complication due to their smaller vasculature6,17. Gruberg et al reported an incidence of 0.40% in women and 0.22% in men who underwent PCI during the same time period6.
Cohen et al10 reported with rotational atherectomy a higher complications incidence associated with procedures to the left circumflex and right coronary arteries, in longer (>10 mm), and eccentric lesions.
Chronic total occlusions (CTOs) which account for about 10% of lesions subjected to coronary angioplasty18,19, constitutes a major cause for wire related perforation. Aziz et al in their study of CTOs observed a perforation in 2.1% of cases20. This was more frequent with long standing CTOs, absence of a visible stump, presence of bridging collaterals, which resist conventional guide wires leading to the use of more aggressive recanalisation techniques21. Recanalisation, if performed through one of the collaterals or through a new channel within the boundaries of the artery too close to the adventitial layers increases the risk considerably.
Calcified coronary arteries also predispose to perforation. This is most likely due to the fact that these lesions are more likely to be treated by debulking techniques. However, it is a well-recognised risk factor for perforation during PCI, irrespective of the equipment used22,23. The site of the perforation in these subsets is more likely to occur at the junction of the plaque border with adjacent vessel wall24. Optimising stent deployment in calcified lesions is presently one of the common mechanisms leading to rupture.
Other complex coronary anatomy, which also warrants use of extra stiff hydrophilic guide wires, debulking techniques and high-pressure balloon inflations and thus predisposes to coronary perforation are vessel tortuosity and ostial lesions14.
Devices and perforations
Guide wire
As discussed earlier, coronary perforation occurred more frequently with debulking techniques than with non-debulking (PTCA and stent) techniques. Ajuni et al3 in their study observed that perforation was caused by a balloon or new device in 74% cases, guide wire in 20% and an indeterminate cause in 6% cases. However, the use of debulking devices is decreasing in most catheterisation laboratories worldwide. As a result, in the future most cases of coronary perforation are expected to occur from guidewires.
Coronary perforation due to guidewire can occur while crossing lesion, due to distal wire perforation or wire fracture. Witzke et al14 reported their experience with 20 guide wire perforations (out of 39 cases) and observed that perforations occurred while trying to cross the lesion with the guidewire in 11 patients (55%), with the distal wire in a small branch in 7 patients (35%) and as a result of wire fracture in 2 patients (10%). Of these cases, perforations occurred with the use of hydrophilic guidewires in 50% of the patients. Of note, ten out of the 20 guidewire perforations (50%) occurred in patients receiving GP IIb/IIIa antagonist agents.
Guidewire perforations such as those that might occur during attempted revascularisation of CTO infrequently lead to cardiac tamponade, except in instances when patients have been pretreated with platelet receptor antagonists. Distal migration of the guidewire in the presence of GP IIb/IIIa inhibition is also an important factor for coronary perforation.
Non-debulking techniques
During PTCA, perforation may occur as a consequence of balloon advancement, balloon inflation or balloon rupture. Since PTCA results in dissection and stretching of the vessel wall, oversized balloons (balloon/artery ratio >1.2) may extend these dissections through the adventitia resulting in vessel perforation. Balloon rupture particularly those associated with pinhole leaks (as opposed to longitudinal tears), creates high-pressure jets that increase the risk of dissection and perforation.
With the advent of the stent era, the use of oversized balloons for predilatations prior to stent implantation has now been given up. Most of the present day scenario of coronary perforations related to PTCA is due to balloon rupture.
The cutting balloon is a non-compliant, balloon catheter equipped with three-to-four microtome-sharp atherotomes. When used appropriately, it is safe and easy to use, with a high immediate success rate and few complications, provided oversizing is avoided. The cutting balloon has proved to be beneficial in treating difficult complex lesions in the coronary arteries. Its application in de novo coronary arterial lesions and in-stent restenosis is still under discussion. Theoretically, this device induces a smaller degree of vessel wall injury localised to the area of incisions and sparing the inter-incisional segments; however, this postulated reduction in restenosis rates has not been confirmed in clinical practice.
Intracoronary stenting can also lead to perforation from use of stiff guide wires, oversized compliant balloons (for stent delivery), high pressure balloons (for optimal stent expansion) or from subintimal passage of the stent into a vessel with severe dissection. Angiographic features associated with stent-related perforation are complex lesion morphology, small vessel diameter, oversized stents (stent/ artery ratio 1.4±0.1), tapering vessel, and recrossing dissections.
Debulking techniques
The incidence of coronary perforation commonly regarded as a very rare complication of balloon angioplasty, is much higher in the era of more invasive interventional devices, particularly plaque-ablative procedures. The overall incidence is 5 to 6 times higher than with the use of balloon angioplasty alone. Devices that alter the integrity of the vascular wall by tissue removal (DCA), pulverisation (rotablation) or ablation (ELCA) can lead to perforation.
With rotablator morphologic features associated with perforation include lesion eccentricity, lesion length > 10 mm and vessel tortuosity6. Oversized devices especially when used to treat bifurcation lesions and lesions located in severely angulated vessel segments substantially increase the risk of perforation.
Adjunctive therapy
Several large placebo-controlled randomised trials of intravenous platelet GP IIb/IIIa blockade therapy during PCI have shown a decrease in major adverse ischaemic outcomes (particularly in diabetic patients and in those where an occluded vessel is treated)25-27 following administration of this adjunctive pharmacotherapy28-31. The convergence of this adjunctive antiplatelet therapy potentially increases the risk for life threatening bleeding following coronary artery perforation.
Though there is an increased of cardiac tamponade following a perforation in patients receiving GP IIb/IIIa antagonists the incidence of perforations observed in various studies was comparable to that observed in series reported prior to the widespread utilisation of these platelet receptor blockade and stenting2-4,7,32. Thus surprisingly no association existed between abciximab use with either the incidence or the angiographic classification of coronary perforation.
Colombo et al33 also concluded that occurrence of coronary perforations was not affected by the use of these agents (0.26% of the patients treated with these agents vs. 0.3% of those who were not). This incidence of perforation in patients who received GP IIb/IIIa inhibitors was similar to that reported with the use of abciximab in the EPIC, EPILOG, CAPTURE and EPISTENT trials29,34,35. In another series, Stankovic et al reported13 a non-significant trend for a higher incidence of perforation with the use of GP IIb/IIIa agents.
It is well known that most operators would use IIb/IIIa inhibitors mainly in settings where the risk of perforations is very low with the opposite in risky situations. Thus this selection bias should be taken into account when discussing IIb/IIIa usage and perforations hence no conclusion can be drawn at this regard.
When is does occur, possible explanations for the association of perforation with the use of GP IIb/IIIa agents may be that they unmask a minimal vessel tear and convert it into overt perforation. Majority of the perforations that were associated with this agent required pericardial drainage and most of these patients developed clinical signs of tamponade after a delay of more than 2 hours after the procedure.
Classification
Ellis et al4 defined a classification for perforations (Table 2).

This may range from mere vessel puncture by the guidewire resulting in minimal dye staining without adverse haemodynamic consequences, to “wire exit”, to vessel rupture followed by brisk extravasation of blood and dye into the pericardial space leading to tamponade and abrupt haemodynamic collapse. Interestingly, the Ellis Type I perforation is angiographically identical to the previously described NHBLI Type C dissection, reinforcing the notion that a continuum exists between dissection and perforation. Ajluni et al3 reported that the relative proportion of the 3-types of perforations were 31%, 50% and 19% respectively.
This classification is important as the major adverse clinical outcomes are related to the angiographic classification of perforation. Type II perforations, when treated with prolonged balloon inflation is usually associated with a low incidence of adverse sequelae (death, myocardial infarction or tamponade). Type III perforations on the contrary are associated with the rapid development of cardiac tamponade in majority of cases, a need for urgent bypass surgery and high mortality. This type carries a poor prognosis despite percutaneous and surgical treatment. Type III “cavity spilling” perforations, however are associated with less catastrophic consequences36.
Diagnosis and outcome
Coronary artery perforation can result in pericardial haemorrhage and cardiac tamponade, fistulae to the left ventricle or the right ventricle or coronary arteriovenous fistulae. Clinically coronary perforation is associated with a high incidence of death (0-9%), MI (4-26%), emergency surgery (24-36%) and blood transfusion (34%)3,4,7. If perforation occurred when GP IIb/IIIa inhibitor was used, the risk of death was two-fold higher37.
The diagnosis of type 3 perforations is obvious by visualisation of contrast extravasation, which warrants imminent treatment. However perforations manifesting as tamponade is usually confirmed by echocardiography or occasionally by detection of tamponade physiology while in the laboratory. Fluoroscopy may reveal immobile heart borders and one can proceed with pericardiocentesis immediately without echocardiography, with rapid restoration of normal blood pressure.
In contemporary practice, cardiac tamponade is a rare complication of PCI. Patients who develop cardiac tamponade in the catheterisation laboratory almost always have coronary artery perforation. In these patients the diagnosis is usually obvious, but other causes of hypotension such as haemorrhage, ischaemia and/or infarction, and anaphylaxis must also be excluded.
When cardiac tamponade is suspected, a right atrial catheter should be placed immediately to allow monitoring of central venous pressure, and echocardiography should be performed to confirm the diagnosis38,39. Excessive reliance should not be placed on right-sided cardiac collapse, and time constraints usually limit detailed evaluation and imaging in a critically ill patient. Although most patients have evidence of pericardial effusion, some may have only a small volume of blood in the pericardial space despite profound haemodynamic instability.
The time duration of manifesting with tamponade can vary. Some perforations are angiographically apparent (especially if the wire was placed in a small branch) and may go undetected during the interventional procedure only to manifest 8-24 hours later with the sudden appearance of cardiac tamponade. This diagnosis should remain high on the list of the differential diagnosis of post-PCI hypotension. Clinical features, which should make one suspicious apart from, unexplained hypotension is chest pain, dizziness or severe bradycardia40. Because some of these late-presenting cases may be due to small coronary perforations, which are often amenable to percutaneous treatment, coronary angiography needs to be performed in all cases to define the underlying cause for tamponade13,38-41. Retrospective review of the angiograms usually does not help in the late presentations of tamponade if no evidence of dye extravasation from the coronary vessel was seen earlier.
Bypass graft perforation may result in chest or mediastinal haemorrhage but cardiac tamponade is unusual due to partial pericardiectomy during bypass surgery, pericardial adhesions and locations of most bypass grafts outside the pericardium.
Another form of perforation leading to tamponade occurs as a consequence of the use of a temporary pacing wire, and interventional cardiologists should be aware of this cause of pericardial effusion and tamponade in patients undergoing PCI41. Though there are no studies done, estimation of the saturation of the pericardial blood can be useful to differentiate between a coronary perforation (arterial saturation) from a right ventricular perforation (venous saturation) due to a pacing lead.
Not infrequently, however, the cause for tamponade is unclear even when the patient is taken back to the catheterisation laboratory.
Prevention
Guidewire positioning
During all percutaneous interventions the tip of the guide wire should advance smoothly beyond the stenosis and retain the torque response. If there is buckling of the guidewire, restricted tip movement or resistance to guide wire advancement the wire may be subintimal and should be withdrawn and repositioned.If there is any concern that the balloon catheter may have entered a false lumen a gentle contrast injection may be delivered through the central lumen of an over the wire balloon after removing the guide wire. Persistent contrast staining indicates that a false channel has been entered and requires withdrawal and repositioning of both the guide wire and balloon.
Wire advancement and distal parking needs a cautionary note especially with the use of glycoprotein IIb/IIIa inhibitors. This applies in the treatment of chronic occlusions where the potential for wire exit is likely to be higher than in a conventional case. A safety approach is to withhold the administration of GP IIb/IIIa the occlusion has been safely crossed with the guidewire and the operator is confident that the tip of the wire is seated intra-luminally in the distal bed of the vessel. Caution should also be exercised in positioning the tip of the guidewire distally, particularly if a hydrophilic wire is used.12
Balloons
Non-compliant balloons may have an important role in the prevention of perforations. Despite the fact the no specific study ever evaluated this area, it is important to point out that compliant or oversized balloons are frequently used to overcome resistant lesions instead of using a dedicated non- compliant balloon.
Device sizing
In some studies3,7 oversized devices (device to artery ratio >0.8 for ELCA and rotablator: balloon to artery ratio >1.2 for PTCA) were found to be important correlates of angiographic perforation. Hence high-risk lesions (e.g. bifurcation, angulated stenosis, total occlusions) are best approached using balloon to artery ratios of 1.0 for PTCA and device to artery ratios of 0.5-0.6 for lasers and rotablator. With these debulking devices it may be prudent to achieve further lumen enlargement by adjunctive PTCA rather than upsizing to a larger device. It is important to take into consideration that some severely calcified lesions will not expand even at high pressure. In these cases, a conservative strategy may be more appropriate than an obstinate pursuit of an optimal result.
An important caveat to prevent a perforation is to appropriately pre-treat a calcified lesion with rotational atherectomy rather than insisting with an aggressive post-dilation sometimes utilising an oversized balloon in an attempt to obtain complete stent expansion. Among the devices sometimes found to be associated with perforations the cutting balloon especially when used to dilate a calcified lesion when the calcium is asymmetrically distributed needs a precautionary note.
Despite the fact the no specific study ever evaluated this area, it is important to point out that compliant or oversized balloons are frequently used to overcome resistant lesions instead of using a dedicated non- compliant balloon.
Other consideration
DCA is not recommended for treatment of dissections due to the risk of perforation and the reliability and effectiveness of the stents. Stent related perforations may be avoided by meticulous attention to balloon sizing and stent position. Stents should not be used when the distal extent of a dissection cannot be identified angiographically.
Treatment
Treatment of coronary perforation in the current PCI era, requires early detection, angiographic classification, and immediate balloon occlusion of coronary vessel extravasation and relief of haemodynamic compromise. Hypotension from tamponade physiology usually responds rapidly to percutaneous pericardiocentesis.
As mentioned earlier guide wire perforations rarely result in adverse sequelae except in some patients who are pretreated with GP IIb/III a receptor antagonists. In contrast perforations caused by balloons, atherectomy devices or lasers may result in haemopericardium and haemodynamic collapse particularly if the pericardium is normal. Regardless of the cause if coronary artery perforation does occur during PCI, initial management should focus on sealing the perforation as quickly as possible to prevent accumulation of blood within the pericardial space and haemodynamic stabilisation.
Non-operative management
Prolonged balloon inflation
As pericardial effusion and tamponade are more likely to occur in type II and III perforations, immediate occlusion of the perforated vessel should be accomplished by prompt and prolonged balloon catheter inflation at the perforation site to prevent further blood extravasation. A balloon (balloon to artery ratio= 0.9-1.0) should be immediately positioned at the site of contrast extravasation (even prior to pericardiocentesis, placement of an IABP or CPR) and inflated to 2-6 atmospheres for at least 10 minutes. If sealing is incomplete a second low pressure inflation should be performed for 15-45 minutes using a perfusion balloon catheter if possible. Perfusion balloon catheters in spite of prolonged balloon occlusion maintains distal myocardial perfusion and avoids myocardial ischaemia. Perfusion balloons thus occludes the hole, attempts to seal the defect, and at the same time permits distal vessel perfusion. Prolonged balloon inflations (and pericardiocentesis if needed) may avoid the need for surgery in 60-70% of patients who develop coronary perforations.
Pericardiocentesis
Echocardiography should be performed at the first sign of perforation if possible.If pericardial haemorrhage is evident immediate pericardiocentesis is performed. If haemodynamic collapse occurs secondary to perforation, pericardiocentesis should be immediately performed after positioning the inflated balloon across the perforated segment. Multiple side-holes catheter should be placed in the pericardial cavity for continuous aspiration and monitoring.
Reversal of anticoagulation
Initial efforts to seal the perforation usually occurs while the patient is anticoagulated. Most interventionalists recommend immediate administration of protamine to partially reverse the effects of systemic heparinisation when free perforation occurs. If contrast extravastion persists despite prolonged balloon inflations, incremental doses of protamine to achieve a partial thromboplastin time of less than 60 seconds or an ACT of less than 150 seconds should be administered. The use of protamine in patients with coronary perforation post-PCI seems to be safe42 with no incidence of vessel thrombosis.
Platelet GP IIb/IIIa inhibitors should be discontinued once perforation occurs. Abciximab effects can be reversed by platelet transfusions (6-10 units) but there is no antidote for eptifibatide or tirofiban. However in the presence of normal renal function, infusions of small molecule GP IIb/IIIa inhibitors such as eptifibatide and tirofiban may be stopped with prompt reversal given their short half-lives.
Covered stents
In the past few years, covered stents have become an alternative to surgery when conservative approaches fail. Some case reports have shown promising results with autologous venous-covered stents43- 45. However, this strategy is technically demanding and may be difficult to perform in emergency situations like Type III perforation. Of note, the time interval from vein harvest to covered stent deployment has been reported as between 20 and 45 minutes.
In contrast, polytetrafluoroethylene (PTFE)-covered stent implantation is easy, rapid, and does not require extensive training. The Jomed Covered Stent Graft (JCSG; Jomed International AB, Helsingborg, Sweden) is a balloon-expandable, slotted-tube stent comprising of a layer of ultra-thin (75 µm) PTFE graft material sandwiched between two stents of reduced wall thickness. Briguori et al46 in their experience observed that PTFE-covered stents successfully sealed 91% of coronary perforations after other conservative approaches had failed. Compared with the non-PTFE group, PTFE-covered stent significantly reduced the need for emergency surgery. Time to deploy the PTFE-covered stent and, subsequently, to seal the perforation is also relatively short. Though none of the patients effectively treated with the PTFE-covered stent implantation experienced any adverse cardiac events, at follow-up the angiographic restenosis was 29%. It is also reported that neointimal proliferation occurs predominantly at the edges, which are not covered by the membrane47. Although there may be a barrier through the stent, tissue response via the ends of the stent may occur. In the same way, endothelisation after implantation may occur only via the ends of the stent. This has been shown in animal models47, suggesting that this stent might need a longer course of combined antiplatelet therapy compared to other stents.
The lack of late thrombo-occlusive events in the PTFE group could be due to (1) the frequent use (50%) of intravascular ultrasound to assess proper stent implantation, (2) the routine use of final high pressure balloon inflation and (3) the prolonged (3 months) antiplatelet administration after the procedure. In the absence of an IVUS, angiographic optimisation is performed with high-pressure balloon dilation to achieve <20% residual stenosis by visual estimate.
However, there is a need for a small (2.5 mm diameter) stent to deal with perforations occurring in small vessels. It is important for this stent to have an enhanced flexibility and a low profile compared to the currently available JOMED covered stent. Other forms of sealing perforated vessels described prior to the introduction of the covered stent include the use of a makeshift stent sandwich.48
Embolisation
Coil-induced occlusion49 is a reasonable therapeutic strategy in selected cases of coronary artery perforation such as persistent perforation in poor candidates for surgical repair due to small vessel rupture, distal location, limited myocardial territory in jeopardy, initial chronic total occlusion or other clinical situations precluding surgery. Guide wire induced perforation of the distal coronary artery may also be treated by gelfoam injection via an infusion catheter.50
Other devices that have been tried in isolated case reports include pre clotted autologous blood clot51.
Successful sealing of the perforation have been reported by injecting polyvinyl alcohol into the distal coronary bed52. Fischell et al53 in their study sealed these tertiary perforations by injecting thrombin at a concentration of 50-100 IU/ml with a delivery of 2-5 ml followed by a 10- to 20-min balloon inflation (to allow enough time to create good cross-linking of the fibrin clot and to prevent retrograde effects of the activated thrombin). Thrombin promotes rapid and localised thrombosis of the tertiary-branch vessel without proximal propagation of clot.
Monitoring following successful non-operative management
Continuous monitoring of the right atrial pressures will allow early detection of ongoing pericardial haemorrhage.If pericardiocentesis was performed during PTCA the drainage should remain in place for 6-24 hours. Serial echocardiography should be performed every 6-12 hours to detect re-accumulation of pericardial effusion. If bleeding persists or recurs the patient should be referred for early surgery.
Operative management
If the perforation is large, associated with severe ischemia or if haemodynamic instability or perforation persists despite non-operative measures, emergency surgery should be performed to control haemorrhage, repair the perforation or ligate the vessel and bypass all vessels containing significant stenosis. If possible a perfusion balloon catheter should be positioned and inflated at low pressure while the operating room is being prepared with intermittent flushing of the central lumen with heparinised saline to prevent clotting and ensure antegrade blood flow.
Operative management is required in 30-40% cases of patients who develop perforation. In-hospital morbidity and mortality is high in these patients especially in the elderly who developed cardiac tamponade. In an earlier mentioned study6 patients who underwent emergency surgery with either pericardial window and/or bypass surgery had a worse outcome, with a higher in-hospital mortality than patients who were treated medically or with emergency pericardiocentesis, reflecting the severity of the perforation and rapid haemodynamic deterioration that required drastic measures.
Cavity spilling/undetermined source
Patients who demonstrate cavity spilling have favourable outcomes with conservative therapy. These patients may respond to prolonged perfusion balloon inflation or the vascular communication may even spontaneously close in follow-up.
Conclusion
The incidence of coronary perforation fortunately remains low in the current device era; it occurs more frequently with debulking devices and often as a consequence of guidewire migration and injury in the presence of GP IIb/IIIa inhibition. Nevertheless, its outcome is not affected by the use of GP IIb/IIIa antagonists. Treatment of coronary perforation requires early detection, angiographic classification, immediate occlusion of coronary vessel extravasation. The poor outcome observed in patients with an undetermined site of coronary perforation or unrecognised perforation suggests that any delay in the diagnosis or institution of appropriate therapy for coronary perforation diminishes the likelihood of a good outcome.

