Abstract
Objective: A comparison of aspiration catheters that have been approved for real-world use was carried out in vitro.
Background: Myocardial damage occurs during therapeutic aspiration of thrombus. The relative efficiency of aspiration may be important in this regard.
Methods: Using saline and human clot, nine aspiration catheters were compared Thrombuster III®N (6 Fr and 7 Fr), ZEEK (6 Fr), Rebirth (7 Fr), Eliminate™ (6 Fr and 7 Fr), Pronto (6 Fr), and Export® (6 Fr and 7 Fr). Tracking was assessed from the resistance required to pass the catheter through a vessel model. Pushability was determined from the difference between the load at the hand piece and tip of the catheter during advancement through the vessel model.
Results: The Thrombuster III®N (6 Fr and 7 Fr) showed significantly better aspiration performance, although the ranking order of the catheters was not the same for saline and clot. The Thrombuster III®N also showed the best tracking with low resistance and was the easiest catheter to advance, as evaluated based on pushability.
Conclusions: In the present in vitro evaluation system, the Thrombuster III®N performed better than other catheters.
Introduction
After percutaneous coronary intervention for acute coronary syndrome, unstable states that are often difficult to manage can occur such as no-reflow and slow flow1-4. The main cause of these phenomena is distal embolism by thrombus or ruptured plaque, so it is important to develop a treatment strategy that prevents such embolism. There have been a few reports about good results obtained by clinical use of thrombectomy devices in the acute stage5,6, but the speed of thrombus removal seems to be important. Accordingly, we performed an in vitro comparison of nine types of thrombus aspiration catheters.
Methods
Aspiration catheters
The catheters investigated could all be used with a 6 Fr or 7 Fr guide catheter. All of them were monorail catheters with a rapid exchange mechanism. A total of 9 catheters from 6 manufacturers were assessed: Thrombuster III®N 6 Fr and 7 Fr (Kaneka-medics), ZEEK 6 Fr (XEON), Rebirth 7 Fr (Goodman), Eliminate™ 6 Fr and 7 Fr (Clinical Supply), Pronto 6 Fr (Vascular Solutions), and Export®6 Fr and 7 Fr (Medtronic). The features of each of these aspiration catheters are compared in Table 1.
In vitro aspiration model (Fig. 1)
Aspiration was compared using normal saline and human blood clot. The weight of the aspirate per unit time was determined by using an electronic balance. Saline was used to determine the maximum performance (Fig. 1a). Human blood clot was obtained by allowing blood from a healthy volunteer with normal coagulation to stand in a glass container for 6 hours. The clot was then infused into a vessel model made from vinyl chloride tubing 6 mm in diameter (Fig. 1b). In order to obtain uniform suction conditions, a 30 ml syringe was used for aspiration. Each test was repeated 10 times, with five measurements, each being done by 2 experimenters under the same conditions.
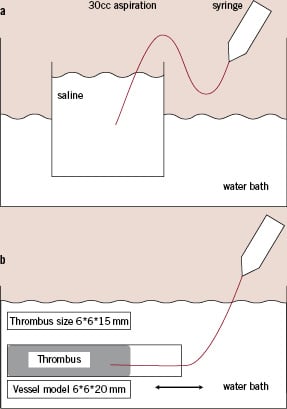
Figure 1. The aspiration model. a. Aspiration of saline. b. Aspiration of human blood clot.
Assessment of tyracking and pushability
Evaluation of tracking was done by using a continuous curve vessel model with 7.5-30 mm radius of curvature, which consisted of a polyethylene tube with an inner diameter of 3 mm. A load meter was used to determine the resistance at the time of passage through the model vessel (Fig. 2a). Catheters were advanced at 20 mm/sec under computer control over a total distance of 150 mm.
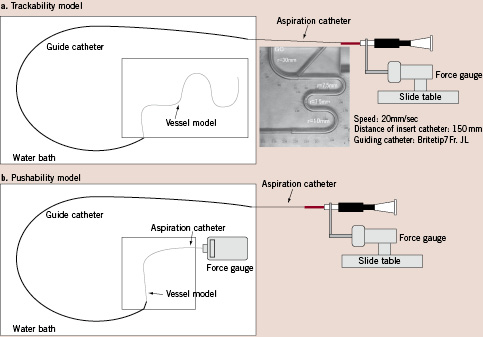
Figure 2. The vessel models. a. Tracking model. b. Pushability model.
Evaluation of pushability (Force transmission)
A catheter was inserted into a curved vessel model made of polyethylene tubing 3 mm in inside diameter. Force transducers were attached to the catheter tip and to the hand piece. Then the force applied to the hand piece and transmitted to the catheter tip was measured while the catheter was advanced under computer control. Force transmission was evaluated as the difference between the force measured at the hand piece and that at the catheter tip using 500-gf and 200-gf force transducers, respectively. The curved vessel model was constructed by creating a 90-degree bend at a site beyond the guide catheter outlet. The catheter was passed through the curved segment to the distal end of the model vessel fixed to the force transducer. Part of the curved model vessel 100 mm proximal to the catheter hand piece was placed in a large water bath warmed to 37°C. The computer-controlled driver on which the catheter hand piece was mounted was programmed to create reciprocating movement of the catheter along the long axis of the model vessel, with the amplitude increasing by 2 mm increments stepwise from 2 mm to 10 mm. Measurement of force transmission was done in triplicate after each increment in the reciprocating movement of the catheter. Five experiments were performed with each catheter tested, and the force transmission rate was calculated as the mean value for these experiments (Fig. 2b).
Statistics
Results are expressed as the mean±standard deviation. Fisher’s direct probability test was used for comparison between groups, with p<0.05 indicating a significant difference.
Results
The aspiration of saline per unit time by each catheter is shown in Fig. 3. Among the 6 Fr catheters, Thrombuster III®N 6F (16.20±0.20) showed the strongest aspiration, followed by the ZEEK (14.69±0.28), Eliminate (13.88±0.15), Pronto (13.22±0.13), and Export® (12.87±0.15), which were significantly weaker (Fig. 3a). Among the 7 Fr aspiration catheters, Thrombuster®7F (25.67±0.71) was also the strongest, followed by Eliminate (22.99±0.18), Rebirth (22.65±0.15), and Export® (22.01±0.20) (Fig. 3b). Regarding aspiration of human blood clot, the Thrombuster III® N 6F (0.19±0.05) was the most effective of the 6 Fr catheters, followed by Pronto (0.18±0.05), Eliminate (0.16±0.07), ZEEK (0.15±0.05), and Export (0.11±0.07) (Fig. 4a). Among the 7 Fr catheters, the Thrombuster III® N 7F (0.36±0.05) was again the most effective, followed Eliminate (0.31±0.04), Export® (0.30±0.02), and Rebirth (0.29±0.05) (Fig. 4b). In each device, the significant difference did not have the human clot. Thrombuster III®N compared with other device and showed the significant trend.
Figure 3. Aspiration of saline. a. 6 Fr catheters. b. 7 Fr catheters.
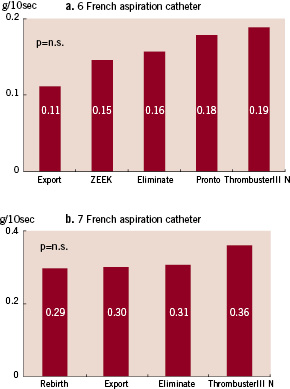
Figure 4. Aspiration of human clot. a. 6 Fr catheters. b. 7 Fr catheters.
The results of the tracking experiment are shown in Fig. 5. The resistance measured after a catheter was inserted into a vessel model was assumed to be related to changes of tracking in the curved model. Among 6 Fr catheters, the Thrombuster III® N 6 Fr, Eliminate, and ZEEK could reach the end of the curved model blood vessel. In particular, the Thrombuster III® N 6 Fr was showed a low resistance (Fig. 5a). Resistance was high for the Pronto and excessive pressure was required, resulting in a broken catheter. Among the 7 Fr catheters, only the Eliminate could reach the end of the model vessel. Judging from the resistance and insertion distance, the Eliminate showed the best functionality, followed by Thrombuster III® N, Rebirth, and Export® (Fig. 5b). To assess pushability, transmission of force was assessed from the difference between the pressure at the catheter tip and that at the hand piece. When the maximum pressure was applied to the 6 Fr aspiration catheters, the Thrombuster III® N 6 Fr showed the highest force transmission, followed by Export, ZEEK, Eliminate, and Pronto (Fig. 6a). Among the 7 Fr catheters, Thrombuster III® N was also the best, followed by Eliminate, Export®, and Rebirth (Fig. 6b).
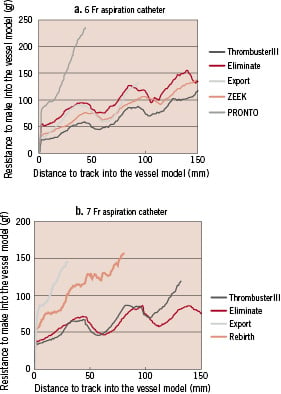
Figure 5. Comparison of trackability. a. 6 Fr catheters. b. 7 Fr catheters.
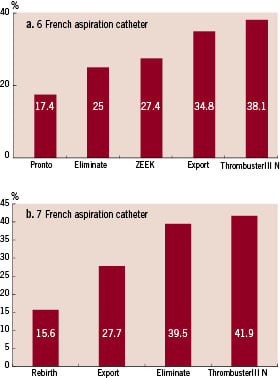
Figure 6. Comparison of pushability. a. 6 Fr catheters. b. 7 Fr catheters.
Discussion
The efficacy of coronary thrombectomy using the TEC7,8, AngioJet9,10, and Hydrolyser11 catheters has been reported in patients with acute myocardial infarction.
Various kinds of aspiration catheters have come to be used, which require complicated techniques and expensive equipment, but examination of clinical usefulness has not been performed sufficiently. The RESCUE™ system12 was the first of these devices, and various other devices with improved operability and aspiration performance have come into clinical use. Under ideal fluid conditions, the volume aspirated increased as the catheter lumen became larger. These results for aspiration volume in relation to catheter lumen were consistent with the following hydrodynamic formula.
The flow rate (Q) of a fluid in a tube with a circular lumen is proportional to the 4th power of the pressure difference (delta P) between the catheter tip(r), the proximal catheter, as shown below, and is in inverse proportion to the viscosity (μ) of the aspirated material and the length (L) of the catheter.
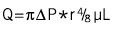
The parameter that influences the flow rate most in the above-mentioned formula is the radius of the catheter lumen, so aspiration of
7 Fr catheters was greater than by 6 Fr devices. The amount of saline aspirated was influenced by the luminal area in each group. However, when human blood clots were aspirated, the results were different from those obtained with saline and aspiration by catheters with almost the same luminal area was variable. Some catheters aspirated human blood clots effectively, despite aspirating only a small amount of saline. In this case, the rigidity of the aspirated material (shape and deformability) and its surface properties related to frictional resistance, as well as the shape of the catheter lumen and differences in frictional resistance related to the catheter material, appeared to influence the results. The Thrombuster® was effective at aspirating human blood clot, and this appeared to be related to its lumen being round, the use of high-density polyethylene with low frictional resistance for the distal shaft, and the lumen of the proximal shaft being larger than that of the distal shaft (allowing clots to slide easily). Based on these findings, in addition to the luminal area and aspiration capacity, additional factors, such as the material and surface properties of an aspiration catheter, or the use of a coating, may affect the performance. In particular, the Pronto aspirated blood clots far better than saline. This finding suggested that the special tip of the Pronto (Silva Tip) might have promoted more efficient aspiration. To compare operability, differences of tracking and pushability between the catheters was assessed numerically using vessel models. Because different results would be obtained according to the material used for the vessel model, we cannot compare the results directly with the expected performance in the coronary artery. However, our curved vessel model could objectively assess the differences in absolute stresses at the tip of each catheter. Based on our findings, operability can probably be increased by changing the catheter material and inserting a core wire. However, it is difficult to obtain both good tracking and pushability because the requirements are totally different. We do not know how differences in operability may influence clinical practice, but in the treatment of proximal lesions causing acute myocardial infarction, catheters with a large lumen and greater aspiration capacity are more effective than those with good operability. For treatment of peripheral emboli occurring during percutaneous coronary intervention, devices with greater trackability should be used because it is necessary to reach a peripheral target. Pushability is most important when it is necessary to cross lesions with severe calcification. Furthermore, in order to cross a lesion with a high percent stenosis, pushability is also an important feature. The aspiration catheters used in this study showed differences of structure, materials, and surface coating, but there is still room for improvement.
Aspiration of clot is not a complete treatment in itself, and these catheters are regarded as supportive devices for percutaneous coronary intervention. In order to achieve the maximum effect with the minimum effort, catheters tailored for each lesion type and purpose-built suction devices are needed, so further improvement is required.
Conclusion
We constructed an in vitro evaluation system for aspiration catheters, and compared various brands of thrombus aspiration devices with respect to aspiration performance, trackability, and pushability. The catheters showed considerable variation of their characteristics, so it is considered necessary to choose the most suitable one for each lesion while taking the operability of the device into account.

