Abstract
Aims: The long-term outcome of extended dual antiplatelet therapy after percutaneous coronary intervention (PCI) is unexplored. The purpose of the study was to evaluate the 2 year safety and efficacy of continuous dual antiplatelet treatment in patients undergoing primary PCI with paclitaxel-eluting stent (PES) for acute ST segment elevation myocardial infarction (STEMI).
Methods and results: A series of 145 consecutive patients with STEMI underwent primary PCI with PES. All patients received intracoronary high dose bolus and 24 hours i.v. infusion of tirofiban and were then treated with dual anti-aggregation up to 2 years. The incidence of major adverse cardiac events (MACE) was evaluated at 1 month, 1 year, and 2 years during the follow-up period. Overall, the rate of cumulative MACE detected at follow-up was 2.7%, with a 1.4% incidence of mortality and a 0.7% need for target vessel revascularisation. No major bleeding occurred during follow-up (95% C.I. 0-2.6%). Safety analysis revealed that minor bleeding occurred in 5 patients (3.4%) at the time of PCI, and that thrombocytopenia developed in 3 patients (2.1%) with the extended clopidogrel course. During the 2 year follow-up, in-stent thrombosis was seen in only 1 patient who stopped drug treatment against medical advice, but did not occur in patients who remained on dual antiplatelet therapy.
Conclusions: This study shows in a ‘real world’ scenario that sustained dual antiplatelet therapy following primary PCI with PES in patients with STEMI is associated with very good 2 year clinical results.
Introduction
Primary percutaneous coronary intervention (PCI) with drug-eluting stents is a superior therapeutic strategy than bare-metal stent implantation in patients with ST-elevation myocardial infarction (STEMI)1-4. However, an unexpected safety concern related to the potentially catastrophic occurrence of late stent thrombosis has recently emerged5,6, leading to widespread uncertainty regarding the risk of clinical events after the discontinuation of clopidogrel.
Extended dual antiplatelet therapy is currently proposed to prevent this complication7-10, but no previous investigation has focused on the very long-term safety and efficacy of this approach in the real world.
We report here the clinical results of our novel strategy of 2 year dual antiplatelet therapy following primary PCI with paclitaxel-eluting stents (PES) in an all inclusive unrestricted population of patients with STEMI which can represent the daily clinical practice and, therefore, may possess a great generalisability11.
Methods
Study population
Over a 1 year period, in 2003, 145 consecutive patients (mean age: 60±11 years) with STEMI of < 12 hours duration (extended to 18 hours in presence of cardiogenic shock or evidence of continuing ischaemia) underwent mechanical reperfusion of the infarct-related artery with implantation of > 1 PES (Taxus®, Boston Scientific, Natick, MA, USA). All clinical situations and lesion morphologies were considered eligible. The study complies with the Declaration of Helsinki, the informed consent was obtained from all patients and the protocol was approved by the human research committee of our institution.
PCI and adjunct drugs
All patients received a loading dose of clopidogrel 300 mg prior to PCI. At the time of coronary angiography, patients received 500 mg of aspirin and an intravenous bolus injection of 5,000 IU of heparin. During PCI, additional heparin was administered to achieve and maintain the ACT values between 200 and 250 seconds. Stents were implanted directly or by balloon predilation. After the procedure, the arterial sheaths were removed when ACT value was < 180 s with external manual compression for haemostasis.
In all patients, tirofiban was given as intracoronary bolus of 25 µg/Kg body weight (i.e. 2.5 time the standard recommended dose) for 5 min, followed by peripheral venous infusion at a rate of 0.15 µg/Kg/min over a 24 hour period. The high-dose bolus was started immediately before the procedure when an antegrade thrombolysis in myocardial infarction (TIMI) grade 1 flow distal to stenoses was present, or after restoring distal flow obtained by crossing the obstruction with guidewire and/or by balloon predilation12.
Procedural success was defined as: 1) a reduction of luminal diameter stenoses below 30% residual luminal narrowing; 2) restoration of epicardial TIMI grade 3 flow; and 3) TIMI myocardial perfusion flow grade 3. Baseline and post-interventional TIMI flow grade and TIMI myocardial perfusion grade of the culprit vessels were evaluated by 2 blinded independent cardiologists with averaging of the scores if they were not in agreement13. Myocardial blush was graded as follows: grade 0-1: no or minimal myocardial contrast density; grade 1: stain or prolonged persistence of dye on next dye injection; grade 2: bright dye appearance at the end of injection, cleared by next injection; grade 3: normal ground glass appearance of blush14.
Quantitative coronary angiography
Quantitative evaluation of the angiograms was performed in all cases. The operators who performed the evaluations were unaware of the patient characteristics and the stent type used. Digital angiograms were analysed off-line with the use of an automated edge detection system (Cardiovascular Medical System, MEDIS, Leiden, The Netherlands)15. The contrast filled non-tapered catheter tip was used for calibration. The reference diameter was measured by interpolation.
Follow-up
All patients received aspirin 100 mg/day and clopidogrel 75 mg/day throughout the follow-up period. Over the 2 year follow-up period, patients were seen after 1 month, and then at 6 month intervals or earlier if clinically indicated. At discharge, patients were advised to stop aspirin but not clopidogrel only in case of intolerance or in case of an excessive bleeding risk (i.e. surgery)16. Adherence of patients to dual antiplatelet treatment was monitored at follow-up visits throughout the 2 year study period.
End-points and definitions
The primary endpoints were a composite of death from any cause, non fatal reinfarction, target vessel revascularisation and stroke within 30 days and at 2 years. Reinfarction was defined as recurrent chest pain with ST-segment upsloping > 2 mV in at least 2 contiguous leads lasting > 30 min and new elevations of the MB isoform of creatine kinase above at least 3 times the upper limits of the normal range in at least 1 blood sample. Target vessel revascularisation was defined as any repeat revascularisation due to stenoses located on the same epicardial vessel treated during primary PCI. Stent thrombosis was defined as an intra-luminal filling defect with contrast staining on 3 sides, representing total or partial stent occlusion, present at the time of a clinically driven, repeat angiography17. Stent thrombosis was subacute when it occurred between 24 hours and 1 month after PCI, whereas it was defined as late when it occurred >1 month after. Stroke was defined as a neurologic impairment resulting in chronic inability to perform daily activities.
At arrival, all patients were stratified on the basis of the thrombolysis in myocardial infarction (TIMI) risk score18. As a safety analysis, bleeding complications were recorded, and TIMI definitions of minor and major bleeding, along with severe or mild thrombocytopenia were used19.
Statistical analysis
Categorical data were presented as absolute frequencies and percent values. Quantitative measurements were expressed as mean ± standard deviation. Confidence intervals were calculated according to standard methods20. The Kaplan-Meier method was used to estimate event free survival as a function of time. Cases lost at follow-up were included in the analysis and considered as censored at the time of the last observation.
Results
Clinical, angiographic and procedural characteristics
Baseline clinical characteristics are shown in Table 1.
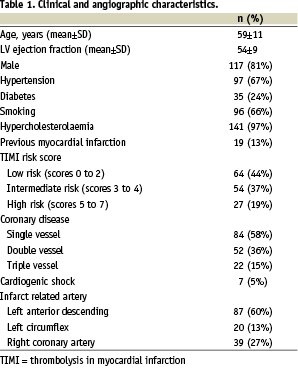
On the basis of the TIMI score at arrival, patients were classified at low risk (44%), intermediate risk (37%), and high risk (19%). Primary PCI was performed in all patients 6 (± 3) hours after the onset of symptoms. Cardiogenic shock was diagnosed in 7 patients (5%). The lesions were located in the left anterior descending in 60% of cases, the left circumflex in 13%, and the right coronary in 27%.
Pre- and post- procedural angiographic characteristics are summarised in Table 2.
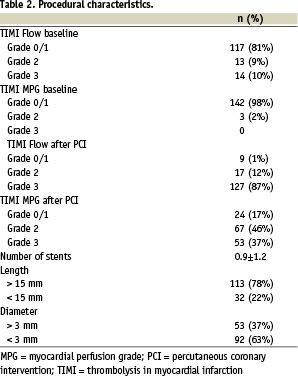
At baseline angiography, TIMI flow 0 to 1 was found in 81% of cases. After PCI, the restoration of TIMI 3 flow was obtained in 88% of patients.
Safety data
The safety profile is shown in Table 3.
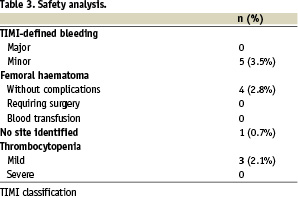
At the time of PCI, 5 patients (3.4%) had minor TIMI-defined bleeding. Four of them (2.7%) had subcutaneous haematoma at the catheter insertion site, without any significant decrease in haemoglobin or any need for blood transfusion. Thrombocytopenia, with a platelet count of < 100.000/mm3, was seen in only 3 patients (2.1%) with the extended clopidogrel course. None of them had a drop in their platelet counts < 50.000/mm3.
It is noteworthy that, no intracranial bleeding event or other major bleeding occurred in the study population over the 2 year follow-up period despite the extended use of dual antiplatelet therapy (zero major bleeding; 95% confidence intervals: 0-2.6%).
Clinical outcome during follow-up
Complete 2 year follow-up was available for all patients but two. Overall, clinical outcomes up to 30 days and to 2 years were favourable (Table 4).
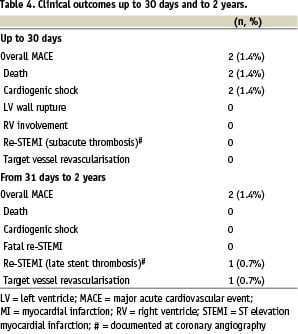
The rate of cumulative MACE detected at follow-up was 2.7%, with 2 deaths and 1 case only of target vessel revascularisation due to in-segment restenosis. Cardiogenic shocks accounted for both cumulative deaths. Cumulative survivals at 30 days, 6 months, 1 year and 2 years are detailed in Table 5.
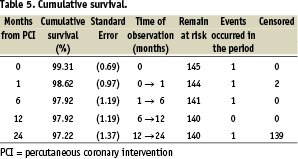
Adherence of patients to our recommendation of an extended dual antiplatelet treatment was found to be very high in all patients. Noteworthy, 96% and 85% of patients of the cohort were on clopidogrel and aspirin at 1 year and 2 year follow-up visits, respectively. At the end of follow-up, 17 patients (12%) had stopped clopidogrel and were on aspirin only, whereas 5 (3%) had stopped aspirin and had remained on clopidogrel only. During the 2 year follow-up, in-stent thrombosis causing a re-STEMI was seen only in 1 patient who stopped drug treatment against medical advice, but did not occur in patients who remained on dual antiplatelet therapy.
Discussion
This study shows, in a ‘real world’ scenario, that sustained dual antiplatelet therapy after primary PCI with PES in patients with STEMI confers optimal 2 year results.
Published data supporting drug eluting stenting in STEMI patients have concluded that they are safe in the setting of primary PCI and reduce the need for repeated revascularisation1-4. These previous studies, however, have followed patients for a relatively short time and therefore do not provide any information on the risk associated with a prolonged treatment with thienopyridine21 and the risk of very late stent thrombosis22. Unfortunately, the use of drug-eluting stents has lately been suggested to be characterised by a shift toward late stent thrombosis even beyond 1 year following PCI23. Available evidences indicate that stent thrombosis continues to occur, particularly in patients with primary PCI24, diabetes, and bifurcation lesions5. In addition, the overall long term incidence is twice that associated with bare-metal stents (1.2% vs 0.6%)25, and it is now clear that premature discontinuation of antiplatelet therapy plays the major pathogenic role26. Although stent thrombosis may occur despite continued dual antiplatelet therapy9, recent findings have motivated the change in common practice among cardiologists to empirically prescribe dual antiplatelet therapy well beyond the 6-9 months initially indicated for a drug-eluting stent23, and two American scientific documents have lately recommended the continuation of dual antiplatelet therapy for 12 months8,9.
Our results show that sustained dual antiplatelet therapy following primary PCI with PES in patients with STEMI is associated with an extremely low incidence of MACE. It is noteworthy that, our strategy of a 24-month clopidogrel course was associated with a frequency of stent thrombosis lower than that recently reported by Spaulding et al3 and Laarman et al4 in STEMI patients treated with drug eluting stents. One should consider, however, that our patients had different risk profiles, all received a glycoprotein IIb/IIIa receptor blocker (i.e. tirofiban) and, most importantly, were advised to continue dual antiplatelet drug regimen over the 2 year follow-up. These differences might explain why in-stent thrombosis occurred exceptionally in our STEMI patients treated with PES, with only one patient experiencing this complication during the 2 year follow-up after withdrawing clopidogrel against medical advice. These findings are of relevance because they rule out the hypothesis that overall stent thrombosis and very late stent thrombosis in drug-eluting stents are more common than currently thought in the so-called ‘real world’ where off-label uses of these devices are more common27. On the contrary, our results demonstrate in a consecutive, non-selected population of STEMI patients that use of PES in primary PCI is both safe and effective even 2 years after the procedure, provided that patients continue dual antiplatelet treatment in the long term. This is in agreement with the results of the BASKET-LATE trial6, and supports the view that until the issue of very late stent thrombosis is further studied patients at higher risk for stent thrombosis should be considered for dual antiplatelet therapy for longer than 12 months after careful review of the risks and benefits10.
Limitations
Our study deals with a single centre registry of PES implantation in patients with STEMI and, therefore, suffers from all the limitations inherent to this particular study design. The relatively low frequency of adverse events during follow-up can be explained in part by the fact that most patients were not at high risk. However, our study is characterised by unrestricted inclusion criteria and a very long term follow-up, thus providing unique data in the ‘real world’11,28. Even when controlled trials are available, carefully designed and prospective registries can provide timely and accurate assessment of the risk/benefit profile of PES in very high risk patient29. Nevertheless, randomised clinical trials are mandatory to definitely ascertain the effects of indefinite dual antiplatelet treatment after primary PCI with PES. In addition, further investigation is needed to evaluate the economic implications of the policy of using a 24 month clopidogrel course. Finally, given that the currently recommended 75 mg maintenance dose of clopidogrel is associated with a high incidence of suboptimal response30, further improvements in our antiplatelet strategies are mandatory.

