Abstract
Aims: Recurrent stenosis and stent thrombosis are still major concerns after drug eluting stent placement which inhibits not only the restenostic process but endothelialisation as well. In contrast, through accelerating rapid endothelialisation and development of an earlier functional endothelial layer, passive coatings have shown encouraging results. The objective of the present study was to investigate the clinical outcome and rate of recurrent stenosis of silicon carbide passive coated cobalt chromium stents (PRO-Kinetic Coronary Stent with PROBIO® coating, Biotronik AG, Switzerland) on restenosis after percutaneous coronary intervention.
Methods and results: Percutaneous coronary stent deployment was carried out in 161 lesions in 145 consecutive patients. The primary combined endpoint was the rate of target-lesion revascularisation (TLR) and late lumen loss; the secondary endpoints were the procedural success and the major adverse cardiac events at 6-months follow-up. Out of 145 patients, 141 were successfully amenable to a silicon carbide coated stent (PRO-Kinetic, Biotronik AG, Switzerland) implantation (97.2% procedural success). At follow-up, the late loss was 0.75±0.71 mm. (in-stent) respectively 0.79±0.72 mm (in-segment), TLR was 4.9% and MACE was 5.6%.
Conclusions: By augmenting rapid endothelialisation and development of an earlier functional endothelial layer, silicon carbide (PROBIO®) as a passive coating on cobalt chromium stents has shown encouraging results relative to success rates, clinical outcome, TLR and late-loss in a cohort of patients with extended coronary artery disease.
Introduction
Coronary stent implantation is the standard therapy during percutaneous coronary intervention (PCI) to prevent early- and late recoil and to reduce the rate of recurrent stenosis. Drug eluting stents (DES) could further reduce the rate of restenosis, but at the expense of delayed re-endothelialisation1-4. But exactly this delayed re-endothelialisation process may be the foremost reason for the increased risk of late stent thrombosis after drug eluting stent, even very late (1-3 years) after stent deployment5,6. This leads to the concept of passive coatings in order to enhancing the endothelialisation process to bypass this risk7-10.
The proliferation of smooth muscle cells as the dominant mechanism of restenosis after stent deployment is suspected to be triggered by interactive processes at the interface between the stent and the biological environment. Today, the majority of stents consist of uncoated 316L medical grade stainless steel or cobalt chromium alloys. But stainless steel and other metals show unfavourable effects when they come in contact with blood constituents like proteins and cells (Figure 1)11,12. These interactions betweens proteins and cells with the implant are the origin of irreversible processes involved in in-stent restenosis: 1) the initiatory platelet aggregation including synthesis and activity of fibrinogen and thromboxane A2, mitogenic activity, leukocyte activation, and complement activation13; 2) endothelial dysfunction promoting vasoconstriction and its sequelae; 3) exposure of medial cells to circulating mitogens14 and increased smooth-muscle-cell proliferation and extracellular matrix formation15. The activation, cleavage or deformation of proteins triggers further biochemical reactions that result in loss of implant functionality. Stent surface modifications (e.g. coatings) should therefore inhibit unwanted interactions between stent and surrounding tissue16.
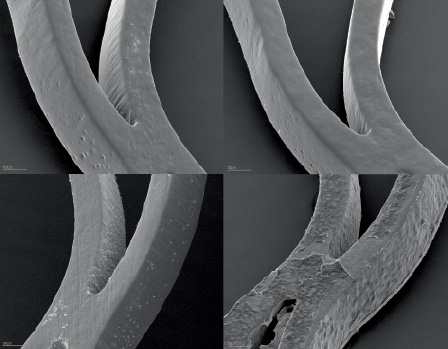
Figure 1. Scanning electron microscopic images. The upper row shows a SiC:H - coated device, the lower row a bare metal stent, before (left) and after (right) blood contact. Note: nearly no fibrin thrombus formation was detected after 120 minutes of blood contact, while the bare nitinol stent showed a dense and organised fibrin network with included platelets and red blood cells.
The concept of Anti-hCD-34 coating capturing circulating CD34+ progenitor cells to the luminal stents struts in order to rapidly differentiating into a functional endothelial layer (Pro-healing concept) have been encouraging7, but comparable results have also been documented in stents increasing early endothelial nitric oxide release in order to promote early endothelial growth and function as strategy against restenosis17-19.
Silicon carbide is an amorphous, hydrogen-rich, phosphorous-doped modification (a-SiC:H) with superior biocompatibility including significant lower fibrinogen and leukocyte adhesion to platelets20-22. Moreover, silicon carbide showed significant lower protein adhesion and increased rapid endothelialisation leading to an improved functional endothelial layer23. In clinical studies, silicon carbide coated stents proved to have significant reduced complication rates when compared to uncoated stainless steel stents24-27, however in the TRUST-study a late catch-up have been recognised at 9-months follow-up in patients with unstable angina28.
Because stents with reduced stent strut thickness maintained radial strength due to cobalt-chromium alloys, as well as demonstrated supplementary effects and encouraging clinical outcomes, we investigated the clinical performance, efficacy, complication rate and incidence of recurrent stenosis after implantation of a new designed silicon carbide coated cobalt-chromium stent in a clinical registry of consecutive CAD patients with indication for percutaneous coronary interventions.
Methods
Study population
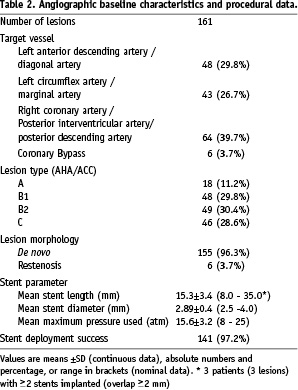
All patients with symptomatic coronary artery disease admitted to the Department of Cardiology and with an indication for percutaneous coronary intervention (intention-to-treat) were enrolled into the study. Enrolment for the PRO-Heal registry took place between February 2006 and June 2007. In this study, patients were eligible for enrolment if they had experienced at least one >70% symptomatic coronary stenosis (visual estimate) in a epicardial coronary artery > 2,25 mm with indications for coronary stent implantation. Patients with known contraindications to aspirin, heparin, or ticlopidine/clopidogrel were excluded from the study. All patients gave written informed consent, and the study was conducted according to the principles of the Declaration of Helsinki. Clinical follow-up at 6-months was requested in all patients enrolled. Due to regulatory and ethical reasons, angiographic follow-up was only requested in those patients with recurrent or new-onset clinical symptoms or in patients with remaining non-target vessel lesions with indication for additional angioplasties.
Definitions
Data collection. Case report forms with pertinent information concerning patients’ demographics, clinical, angiographic, and procedural data were recorded prospectively on standardised forms and entered into a computerised database. We collected demographic and baseline data on the following: gender, age, history of diabetes, left-ventricular ejection fraction, severity and extend of lesion distribution resulting from coronary artery disease, stenosis morphology, actual myocardial infarction (ST-STEMI) and Non-ST-Elevation Myocardial Infarction (NSTEMI), unstable angina pectoris, creatine kinase values, as well as reference coronary diameters of treated coronary vessels, clinical presentation, angiographic findings, procedural success, balloon angioplasty procedure, adjunctive therapy, acute results, procedural complications and hospital course. All events were recorded and maintained in a central cohort database. Stenosis morphology was classified as type A, B1, B2, or C stenosis according to AHA/ACC guidelines29. Angiograms were initially evaluated by the investigators, using visual assessment at baseline, immediately after balloon angioplasty respectively following stent placement. The morphology of the target lesion, presence and degree of calcium, degree of plaque burden, percent of diameter stenosis and thrombolysis in myocardial infarction (TIMI) grade flow were documented. Throughout the study, adverse cardiac and cerebral events were recorded, including death, cerebrovascular accident, abrupt or late vessel closure, re-infarction, perforation, dissection, distal embolisation or no-reflow phenomena as encountered during the procedure and hospitalisation.
Angioplasty protocol
A dose of 100 mg of aspirin was administered before angioplasty and daily thereafter. Heparin was given intravenously in an initial bolus of 7.500-10.000 U, followed by incremental bolus doses to maintain an activated clotting time during the procedure. Intracoronary nitroglycerin was administered before and during the procedure at the operator’s discretion. The size of the balloon and stent (PRO-Kinetic Cobalt Chromium Coronary Stent with PROBIO® silicon carbide coating, BIOTRONIK AG, Switzerland) was selected according to an estimated ratio 1:0 for diameter of stent/reference artery. The length of the stent was chosen on the basis of the length of the target stenosis. In case of non-sufficient deployment of the stent, post-dilatation with a non-compliant balloon was carried out.
Study endpoints and definitions
The primary study endpoint was the rate of target lesion revascularisation (intention-to-treat) and late loss at 6-month follow-up. The secondary combined endpoint was the procedural success and the rate of major adverse cardiac events (MACE). MACE was defined as: 1) cardiovascular death; or, 2) unstable or stable angina pectoris with need for target lesion revascularisation (intention-to-treat); or, 3) myocardial infarction (ST- or non ST-elevation myocardial infarction (STEMI, NSTEMI) with elevation of creatine kinase (CK) levels greater than twice normal laboratory values with any abnormal MB fraction during the follow-up period.
Procedural success was defined as the ability to insert the stent into the target lesion, to achieve final reduction of lumen diameter stenosis to < 30% (after stent deployment), and to obtain TIMI 3 flow in the absence of major in-laboratory and in-hospital complications (death, emergency bypass surgery, development of a new non-ST-elevation myocardial infarction, or ST-elevation myocardial infarction and target vessel revascularisation). Perforation was defined as persistent extra vascular collection of contrast medium beyond the vessel wall with or without associated clinical complications.30 Dissection was considered to be major if it was flow-limiting or associated with death, myocardial infarction, or the need for bypass surgery - and minor if it did not lead to clinical complication.31 Acute closure was defined as reduced antegrade flow (TIMI < l) caused by acute occlusion of the target lesion. Intra coronary thrombus was defined as an intra-luminal lucency, filling defect, or staining consistent with angiographic appearance of thrombus.32 ST-elevation myocardial infarction was diagnosed as the development of ST-elevations on the electrocardiogram and elevation of creatine kinase-MB above laboratory normal values. Non-ST-elevation myocardial infarction was defined as creatine kinase determination of twice the normal, with a positive MB with or without persistent ST-segment or T-wave changes on the post-procedural electrocardiogram. Groin site complications included need for blood transfusion or surgical intervention.
Statistical analysis
Statistical analysis was performed at the Data Management Centre of Genae Associates in Antwerp, Belgium using the SPSS platform (SPSS Inc, Chicago, IL, USA). Categorical variables, described using absolute numbers and frequency of characteristics or event, were assessed by chi-square or Fisher’s test. For comparison of continuous measures described by means with standard deviations, Student’s t-test was used. Changes in TIMI grade, target lesion revascularisation, and percent of diameter stenosis were tested using a Wilcoxon sign rank test. A p < 0.05 was considered significant.
Angiographic analysis
Coronary angiography was performed in a routine manner. We obtained measurements of the interpolated reference diameter, lesion length and diameter, diameter stenosis, and minimal luminal diameter (MLD) before and after the intervention and at follow-up, from angiograms performed after intra coronary administration of nitroglycerin in all patients, where follow-up angio was available. Coronary angiograms were obtained in a routine manner by experienced cardiologists, who had limited information about patients including their laboratory results or previous angioplasty treatment. Quantitative coronary arteriography (QCA) was performed by independent interventionists at the core laboratories of Genae Associates in Antwerp, Belgium using the QAngioXA platform (Medis Medical Imaging Systems B.V., Rotterdam, the Netherlands) with previously stipulated and validated edge-detection algorithms using a catheter for calibration33,34.
Results
Clinical characteristics
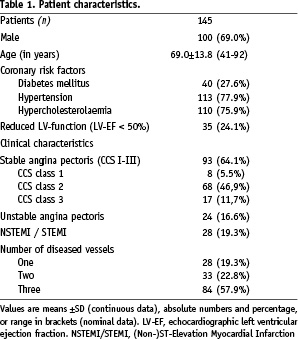
Silicon Carbide coated cobalt chromium stent angioplasty was performed on a total of 161 lesions in 145 patients with a mean age of 69.0±13.8 (range 41-92, 69.0% males). Further baseline characteristics of the total population are shown in Table 1. The baseline morphologic characteristics of the target vessel are expressed in Table 2.
Procedural results
Table 2 depicts related angiographic baseline characteristics and procedural information. Stent deployment success was achieved in 141 patients (97.2%); deployment failures occurred in four patients (2.8%) due to severe calcifications and/or tortousity of the target vessel.
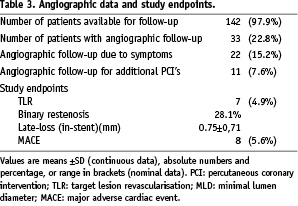
Invasive 6-month follow-up data were available for 33 patients (22.8%). 22 of the 33 patients (15.2%) had invasive follow-up due to symptoms, 11 patients (7.6%) had re-angiography due to additional planned interventional procedures.
Non-invasive follow-up was available in 142 patients (97.9%): One patient got lost to follow-up, two patients died during the follow-up period due to non-cardiac causes. Twenty-two patients (15.2%) had typical or atypical angina pectoris during the follow-up period and were considered for re-angiography, of whom seven patients had significant restenosis with need for re-angioplasty (TLR 4.9%). One patient had unstable angina pectoris 134 days after the index procedure of a 90% stenosis of a postero-lateral bypass graft. Angiographically, a re-occlusion in the stent with additional thrombus (not encountered as stent thrombosis) was diagnosed. No patient required emergency bypass surgery or experienced a procedure-related cerebral vascular accident. Therefore the total rate of MACE was 5.6%.
By QCA, the angiographic in-stent diameter stenosis (DS) was reduced significantly: from 68.9±18.2% to 15.72±9.6% immediately after stent angioplasty (p=0.0061), and to 42.0±23.4% at follow-up (p=0.018*) (*as compared to baseline). The in-stent minimum lumen diameter (MLD) increased significantly: from 0.84±0.48 mm to 2.30±0.62 mm immediately after stent angioplasty (p=0.0089), and to 1,58±0.87 mm at follow-up (p=0.012*) (*as compared to baseline). Therefore the in-stent late loss (LL) was 0.75±0,71 mm (LL in-segment: 0.79±0,72 mm).
Discussion
The optimal behaviour of a stent material regarding its interaction with the environment is achieved if complete endothelialisation with a long-term stable and fully functional layer of endothelial cells is guaranteed within several days. Sound endothelial cells, being the natural interface between blood and the vessel wall, are in a way the perfect coating for a stent. On the one hand, they behave passively with regard to proteins of the coagulation cascade and platelets. On the other hand, they are key players in the mechanisms controlling the growth of smooth muscle cells. Laboratory findings and relatively small clinical studies have disclosed that stent surface rapidly differentiating into a functional endothelial layer can influence the angioplasty-related restenotic process10, which consists of a range of different constituents35. These findings include the fact that a functional endothelial layer inhibits smooth muscle migration and proliferation, and reduces the formation of extra-cellular matrix proteoglycans which provides cellular migration36,37. However no data have yet been published whether stent surfaces may influence the constrictive arterial remodelling following balloon angioplasty (i.e. adventitial collagen accumulation and subsequent thickening) which can be prevented by matrix metalloproteinase inhibition38. But the degree of biocompatibility of the stent surface can induce restenosis on the basis of persistent platelet activation, which in turn inactivates endothelial relaxation factors.
In vitro studies showed that after 24 hours of contact between the CPAE and 316L stainless steel, no continuous endothelial layer had formed (Figure 2a)23. The majority of cells had no contact with their direct neighbour. The single endothelial cells showed marked pseudopodia with lengths of about 1 µm (Figure 2b)23. In contrast to these observations, the situation is completely different on the a-SiC:H coated samples. During the same time and under the same experimental conditions, a continuous endothelial layer covering the whole sample had developed (Figure 3a). The single endothelial cells showed a sound spindle-like shape and the endothelium itself had a characteristic pavement structure including cell-to-cell contacts between neighbouring cells (Figure 3b)23.
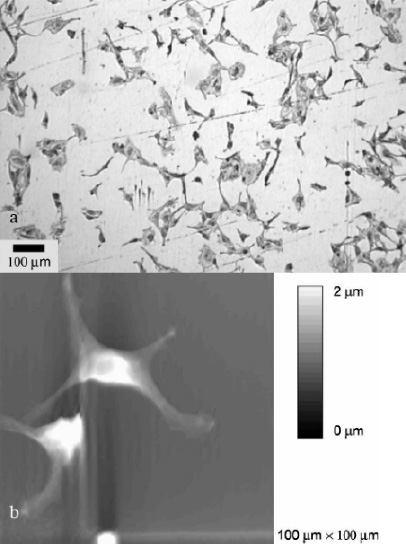
Figure 2. Endothelial cells (CPAE) after 24 hours of contact with 316L stainless steel in modified Eagle medium: (a) Light microscopy image showing only isolated cells on the surface. (b) AFM image showing endothelial cells with marked pseudopodia.
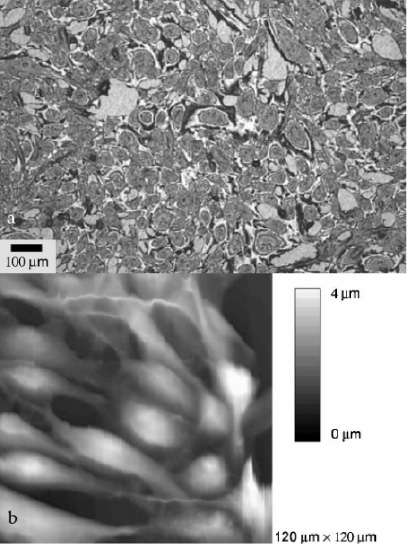
Figure 3. Endothelial cells (CPAE) after 24 hours of contact with silicon carbide (a-SiC:H) coated stainless steel in modified Eagle medium: (a) Light microscopy image showing an endothelial layer covering the silicon carbide completely. (b) AFM image showing endothelial cells with characteristic pavement structure.
Exactly these SiC:H-effects in vitro potentially explain our encouraging clinical results in this study: Through accelerating rapid endothelialisation to an earlier functional layer, SiC:H -coating on ultra-thin cobalt chromium stents leads to less recurrent stenosis after stent placement without increased occurrence of stent thrombosis. In this study, not only the clinically driven TLR (intention-to-treat) appeared very low, but the late loss assessed by QCA was 0.75 mm which is almost exactly the value seen after placement of the Endeavor drug eluting stent39.
Of note, and in contrast to all drug eluting “active” coatings, this favourably low late loss was achieved with a “passive” coating accelerating a rapidly and fully functional endothelial layer without risk of acute or late stent thrombosis (0% stent thrombosis). This includes not only additional safety and a lower risk of stent thrombosis, but also significant medical benefits (and lower costs) due to a substantially shorter dual antiplatelet therapy of just four weeks.
Moreover, the remarkably low restenosis and MACE rate occurred in a cohort of patients who represented a considerably high-risk treatment group with lesions difficult to treat (59% of all lesions were B2- and C-lesions).
But it is in exactly these lesions which are difficult to treat, where due to its low profile, SiC-coating and extremely flexible design, that the PRO-Kinetic stent could be implanted in almost all patients. The documented rate of recurrent stenosis – with or without the necessity for repeat revascularisation procedures – was outstandingly low, even in comparison to published data after drug eluting stent placement in this lesion subset. Moreover, in three of the seven patients with invasive follow-up, TLR appeared in stents, which have been implanted using low deployment pressures (8 atm). This was due to the operators’ discretion and his apprehension of possible vessel perforation in very old, respectively severely calcified lesions.
In the present study, the silicon carbide coated cobalt chromium PRO-Kinetic stent could be deployed in almost all lesions, although, due to its unique flexibility and low profile, it had been used predominantly in difficult and severe lesions. Only in 2.8% of cases was it not possible to deploy this stent in the target lesion.
Limitations of the study
This report concerned analysis of treatment by silicon carbide coated cobalt chromium stent deployment. While it provides intriguing data regarding the safety and effectiveness of the silicon carbide coated cobalt chromium stent in consecutive “real world” patients, it is based on mono-centric data from a non-randomised, observational, study. Despite the considerable success of the silicon carbide coated cobalt chromium PRO-Kinetic stent in these patients, this study offers no proof that similar or improved results could not have been gained with a different therapeutic approach and/or treatment modality.
Summary
Through augmenting rapid endothelialisation and development of an earlier functional endothelial layer, silicon carbide as a passive coating on cobalt chromium stents has shown encouraging results regarding success rates, clinical outcome, TLR and late loss in a cohort of 161 lesions (145 patients) with extended coronary artery disease. Large randomised trials are needed for further investigation.

