Introduction
ClearWay™ RX is a novel therapeutic perfusion catheter allowing localised perfusion of drugs not only into the vessel lumen, but also directly into the vessel wall at low pressure, during coronary intervention.
It combines three procedures in one - occlusion, containment and infusion(OCI). OCI allows for localised delivery of drugs into a targeted area while blood flow is occluded, maximising drug availability without substantial dilution by the systemic circulation. Containment and controlled infusion result in increased residence time of the drug resulting in enhanced bioavailability and overall therapeutic effect.
Abciximab is a glycoprotein IIb/IIIa antagonist that not only inhibits platelet aggregation but also lysis fresh thrombi. Intracoronary (i.c.) administration of abciximab has been shown to be more effective than intravenous (i.v.) infusion1 and local concentration of the drug can be increased by using the OCI technique.
This technology creates a novel approach for localised vascular delivery of existing and new pharmaceutical agents which may prove beneficial, with reduction in systemic complications. It has been used for localised drug delivery to improve perfusion in the setting of high thrombus burden.
Description
The system consists of a perfusion balloon mounted on a rapid exchange monorail platform (Figure 1).

Figure 1. The ClearWay™ RX perfusion catheter.
The semi-compliant balloon is produced from microporous expandable polytetrafluoroethylene (ePTFE) which infuses across the entire surface, without high-pressure jetting. Low pressure inflation of the balloon between 2-4 atm generates sufficient radial pressure to drive the drug into the clot and/or the intima, but is low enough to prevent trauma to the vessel wall. The catheter is designed to be occlusive as temporary arrest of flow improves uptake of drug into the vessel wall, and is generally well tolerated.
Other perfusion catheter systems use a jetting action or micro-needles to force drug deep into or in some cases through, the medial layer of the vessel wall. The ClearWay™ RX catheter provides a less traumatic approach, using a low pressure system (< 4 atmospheres) to deliver drug to the intima. This low pressure technique does not over-extend or damage the vessel wall and it allows the treatment of native vessels, stented vessels and synthetic grafts.
History
The ideal treatment for the management of intracoronary thrombus should:
– penetrate and lyse the thrombus core,
– penetrate the intima at the site of the culprit lesion to achieve meaningful intravascular drug concentration to neutralise the source of ongoing platelet activation (usually a ruptured plaque),
– penetrate the distal microvasculature, to dissolve downstream thrombus,
– have minimal systemic effects,
– be easy to deliver to the culprit lesion,
– be able to deliver the drug in a rapid and controlled fashion,
– be delivered through a system which causes no disruption to the vessel wall.
There is evidence that intracoronary administration of antithrombotic agents improves outcome in patients undergoing angioplasty.
A study by Sezer et al2 randomised 41 patients to i.c. streptokinase or no additional therapy post angioplasty. Cardiac catheterisation was repeated two days later and all measures of microvascular function were improved in the streprokinase group compared to the control group. Although there was no difference in LV function between the two groups, the study was underpowered to detect such a difference.
Abciximab is a potent glycoprotein (GP) IIb/IIIa inhibitor of platelet aggregation and thrombus formation. These properties have been shown to prevent not only thrombus formation but also to promote lysis of fresh thrombus at higher concentration. Wohrle3 demonstrated a significant reduction in MACE at 30 days using i.c. compared with i.v. administration in patients with acute coronary syndrome (ACS) during angioplasty. In a study by Romagnoli,1 there was an enhanced therapeutic effect with local administration of abciximab when flow was compromised by thrombus, allowing for an increased concentration of drug locally. ClearWay™ RX occludes the flow to increase the concentration and maximise the therapeutic effect of the infused drug.
The ClearWay™ RX perfusion balloon has been designed to meet these needs. The catheter is a novel approach for the delivery of therapeutic agents during and after PCI. So far, clinical experience has only been obtained with antithrombotic agents.
Technical specifications
The system is compatible with delivery through a 6 Fr guiding catheter. The balloon is mounted on a monorail platform which is 0.014” guidewire-compatible. Two radiopaque markers identify the edges of the balloon treatment zone. The balloon inflates at 1 atm, but is not fully inflated until 2 atm. The catheter is available in 1.0 to 4.0 mm balloon diameters, with a length of 20 mm, although lengths up to 50 mm are in development. The ePTFE balloon material has a microporous architecture with solid nodes connected by a series of fibrils (Figure 2).
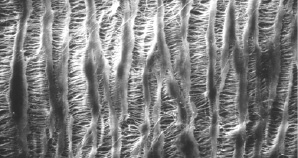
Figure 2. ePTFE balloon material has a porous architecture with solid nodes connected by a series of fibrils.
The pore design allows the balloon to deliver complete circumferential treatment to the site.
Infusate weeps out among the fibrils along the entire length of the balloon ensuring a lubricating fluid layer over the whole surface. The tortuous channels of ePTFE do not allow a direct fluid path to the surface and this prevents the damage induced by a “jetting” effect. The rate of fluid flow can be controlled through the amount of pressure applied by the operator (Figure 3).
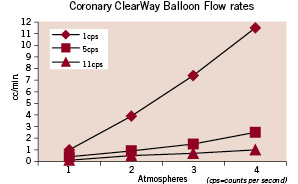
Figure 3. Flow rates through the ClearWay™ RX balloon at several pressures and viscosities.
The catheter is designed to be occlusive. It has been reported that arrested blood flow plays an important role in the uptake of therapeutics into the vessel wall and short periods of occlusion are generally well tolerated.
Indications for use
This perfusion catheter is indicated for localised delivery of agents to the coronary and peripheral vasculature.
The perfusion catheter allows focal drug delivery into a targeted part of the vessel. During balloon inflation, the drug is infused through the microporous balloon pores while blood flow is occluded, maximising drug availability without any dilution by the systemic circulation or washout. This may allow a reduction in dose, compared to conventional i.v. infusions.
The device is not indicated with any specific drug.
The manufacturers recommend use during angioplasty to reduce platelet activation and thrombus formation by administration of anti-thrombotic agents to the stented site. The device can also be used pre-stenting to deliver thrombolytic agent to the thrombus prior to stenting.
As cardiologists, we foresee three main areas for use.
Thrombus
The main indication will be the local delivery of thrombolytic, glycoprotein IIb/IIIa inhibitor or anti-thrombotic agents to improve flow and distal perfusion. Not only will this technique improve local tissue penetration of the drug, but it will also minimise the risks and potential side-effects associated with systemic drug delivery. Intracoronary abciximab has been shown to reduce major adverse cardiac events compared with intravenous administration in patients with acute coronary syndromes undergoing PCI.3 Furthermore, it is well recognised that one of the limitations of intracoronary thrombolytic infusion in the setting of high thrombus burden is that the drug fails to achieve fibrinolysis because it cannot penetrate the core of the occlusive thrombus. Interventionalists have therefore used a combination of balloon inflation (to disrupt the thrombus) and intracoronary infusion of thrombolytic. However, these steps can only be performed sequentially and during balloon inflation, the risk of downstream embolisation with “trashing” of the microvasculature is considerable. The ClearWay™ RX system allows these steps to be performed simultaneously, achieving low pressure inflation but simultaneous drug delivery to the core of the thrombus and into the intima. In addition, even in the absence of visible thrombus, administration of antithrombotic agent at the time of PCI has been shown to reduce the risks of no reflow, acute thrombosis and even restenosis.
Spasm
Severe refractory spasm which fails to respond to bolus administration of intracoronary nitrates may be another indication. Firstly, unlike an intracoronary bolus drug administration, which only delivers drug to the vessel lumen, the ClearWay™ RX system can deliver nitrates directly into the endothelial layer to enhance vasorelaxation. Secondly, the low pressure system will minimise the risk of balloon-induced disruption of the endothelium in an un-diseased part of the vessel.
Prevention of restenosis
Following successful angioplasty, anti-proliferative agents could be delivered directly to the vessel wall through the ClearWay™ RX system. Such localised drug delivery would obviate the need to use drug-eluting stents, and thus remove the potential polymer-related risk of stent thrombosis.
Abcimiximab administration locally increases the drug concentration by binding to platelets and endothelium receptors. It promotes lysis of fresh thrombus and exerts an anti-thrombotic effect by suppression of inflammatory pathways, and may prove beneficial in prevention of restenosis.
A similar approach has been used by Dr Herdeg and co-workers for delivery of paclitaxel to prevent restenosis, albeit with a different catheter system.4 Preclinical use of paclitaxel in pig coronary stents have shown reduced neointimal proliferation, although data from larger trials will be required prior to implementing this technique in humans.
Tips and tricks for delivery
The catheter is removed from its packaging (but not from the balloon protecting sleeve) and connected to a 3-way tap. The side-port is connected to a 10 cc syringe filled with saline which is used to flush the catheter. The remaining port, in line with the shaft of the catheter, is connected to a syringe or inflation device containing the drug of choice. The protective sleeve is removed from the balloon and the device is delivered over-the-wire to the required site, the balloon inflated and the drug delivered through the attached three way tap.
The following are useful tips:
1. Do not dispose of the protective sleeve of the balloon. Flushing of the catheter should be performed with the protective sleeve in situ. If the catheter is removed from the patient and subsequent use is required, replace the protective sleeve and flush the catheter again with the sleeve on, to ensure the balloon profile is kept to a minimum and to prevent the tiny pores becoming clogged with clotted blood outside the patient.
2. Infuse drug through a syringe aligned with the catheter shaft as this can achieve higher pressure inflation and more rapid drug delivery than the side-port.
3. The device is rather bulky, and for tortuous vessels a buddy wire may be required for delivery. If a buddy wire is required, it is worth remembering:
a) that a 6 Fr guiding catheter will have difficulty accommodating two wires and the ClearWay™ RX system, so best to upsize to a > 7 Fr guide;
b) to remove the buddy wire prior to ClearWay™ RX inflation, not only to avoid “cheese-wiring” of the vessel wall by the buddy wire (sandwiched between the inflated balloon and the endothelium), but also to ensure 360 degree circumferential drug delivery.
Preclinical experience
Staining experiments performed in rabbit femoral arteries demonstrated that the ClearWay™ RX catheter primarily delivers drug to the vascular intima, one to two cell layers deep at 2 atmospheres, and five layers deep at 6 atmospheres. Since drugs may differ greatly in viscosity and/or chemical properties, the permeability of the balloon to each drug should be tested on an individual basis.5
In pigs, the ClearWay™ RX catheter was used following successful coronary stenting, to deliver local rapamycin. Examination of the hearts at one hour or three days revealed dose-related tissue deposition of rapamycin into the treated coronary segments similar to that seen with drug-eluting stents (Figure 4).
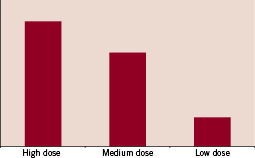
Figure 4. Dose-related rapamycin loading of coronary vessel tissue in porcine coronary arteries (obtained using high pressure liquid chromatography).
The amount of tissue uptake could be controlled by the concentration of drug in the infusion solution dose-dependently.5
Clinical experience
We used the ClearWay™ RX Rapid Exchange therapeutic perfusion catheter to deliver abciximab during primary PCI in a patient with high thrombus burden. A 62-year-old man presented with posterior myocardial infarction to our emergency department. Cardiac catheterisation revealed a 90% circumflex stenosis with thrombus and TIMI 2 flow (Figure 5).
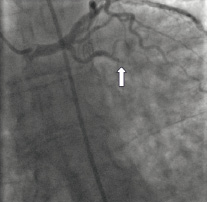
Figure 5. Left coronary angiography demonstrating thrombotic occlusion of the circumflex.
There was atheroma (with thrombus) in the LAD and a 70% RCA stenosis. We proceeded to angioplasty the circumflex. An EBU 4 guiding catheter and an ATW wire was used.
The lesion was predilated with a 1.5x15 mm balloon and 12.6 ml abciximab was infused at the lesion via a 2.5x20 mm ClearWay™ RX balloon (Figure 6).
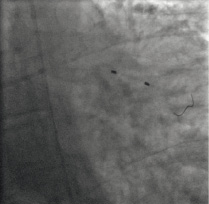
Figure 6. The ClearWay™ RX balloon is seen positioned across the thrombotic lesion, while abciximab is infused through it.
This resulted in visible reduction in thrombus burden and improvement in flow (Figure 7).
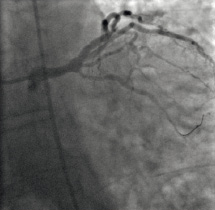
Figure 7. Angiography following ClearWay™ RX removal demonstrated restoration of normal coronary flow and absence of visible thrombus.
The lesion was then stented with a 2.75x18 mm Cypher overlapping with a 3.0x18 mm Cypher stent. A 3.0x12 mm Vision stent was deployed to cover the ostial circumflex lesion. An excellent angiographic result was obtained.
In conclusion, the ClearWay™ RX rapid exchange perfusion catheter can be used to deliver pharmacological agents, in this case, glycoprotein IIb/IIIa inhibitor, to the local lumen and the intima, to successfully treat thrombus in the setting of acute coronary syndrome.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.
Moving image 4.

