Abstract
Aims: OAT randomised patients with an occluded infarct artery three to 28 days after myocardial infarction (MI). The study demonstrated that PCI did not reduce the occurrence of the primary composite endpoint of death, re-MI, and New York Heart Association class IV heart failure in comparison with patients assigned to optimal medical therapy alone (MED). In view of prior literature in similar cohorts showing fewer sudden cardiac deaths and less left ventricular (LV) remodelling, but excess re-MI with PCI, causes of death were analysed in more detail.
Methods and results: Stepwise Cox regression was used to examine baseline variables associated with causes of death. The immediate and primary cause of death did not differ between 1,101 PCI and 1,100 MED patients. One-year cardiovascular death rates were 3.8% for the PCI group, and 3.7% for the MED group, and 0.9% per year for the next four years in both groups. Five of six cases of cardiac rupture occurred in patients undergoing PCI.
Conclusions: In stable post-MI patients with occlusion of the infarct-related artery, PCI did not change the rate or cause of death. The observation that the majority of cardiac ruptures occurred in patients undergoing PCI deserves further investigation.
Introduction
The Occluded Artery Trial (OAT) tested the hypothesis that stable, high-risk patients with persistent total occlusion of the infarct-related artery (IRA) benefit from percutaneous coronary intervention (PCI) with opening of the occlusion within three to 28 days after the index infarction. This “open artery hypothesis” has previously been addressed by studies demonstrating increased electrical stability with fewer sudden cardiac deaths (SCD)1, less adverse LV remodelling with PCI2-5, and the provision of vascular territories that function as collaterals in the instance of future events6,7.
The primary endpoint of OAT was the first occurrence of recurrent myocardial infarction (MI), hospitalisation/short stay unit treatment of New York Heart Association class IV congestive heart failure (HF), or death. After randomising 2,166 patients to PCI vs. optimal medical therapy (MED), the cumulative primary event rate was 17.2% in the PCI group, and 15.6% in the medical treatment group, indicating that PCI did not reduce the composite endpoint8. The rate of death was not different between treatment groups after a mean follow up of 2.9 years, yet trends were observed towards excess reinfarction but improved LV remodelling in the PCI group in a mechanistic ancillary study8. The causes of death analysis in OAT had not been prespecified, and its purpose was to examine whether there were more MI-related deaths in the PCI group.
Methods
Study population
Patients were eligible for enrolment in OAT if coronary angiography, performed on days three to 28 after myocardial infarction, showed total occlusion of the IRA with poor or absent antegrade flow, defined as a Thrombolysis in Myocardial Infarction (TIMI) flow grade of 0 or 1, and if they met a criterion for increased risk, defined as an ejection fraction of less than 50% (assessed by echocardiography, radionuclide ventriculography, or contrast ventriculography), proximal occlusion of a major coronary artery with a large risk region, or both. Qualifying angiograms were reviewed at a core angiography laboratory9. The qualifying period of three to 28 days was based on calendar days; day one was the day of the onset of symptoms. Exclusion criteria were NYHA class III or IV heart failure, shock, a serum creatinine concentration higher than 2.5 mg per decilitre (221 µmol per litre), significant left main or three-vessel coronary artery disease, low threshold or rest angina, or severe ischaemia on stress testing, which was required if the infarct zone was not akinetic or dyskinetic.
Patient recruitment
During two, one-month periods, 4,623 patients with acute myocardial infarction not eligible for the Occluded Artery Trial were recorded in screening logs. Among those patients with ST elevation myocardial infarction logged, 63% underwent primary PCI8. Based on a broad literature search10-16, it is estimated that in contemporary PCI services, approximately 109,000 patients per year would fulfil OAT eligibility criteria in the USA.
Definitions
The immediate cause of death, and disease or injury initiating the train of events leading directly or indirectly to death (primary cause of death) were examined on an intention-to-treat basis.
All deaths were categorised by an independent morbidity and mortality classification committee (MMCC, ≥ two reviewers per event), which was blinded to treatment assignments with reporting of the primary and immediate causes using standardised definitions.
Patient follow-up
For this analysis, we have updated the database using all patients enrolled through June 30, 2006 (N=2201) and included all follow-ups through March 30, 2007. Compared with the database underlying the primary OAT report8, we have added 35 patients enrolled in an extension phase of the viability ancillary study in 2006. The average follow-up is now 3.2 years, compared with the original OAT cohort follow-up of 2.9 years.
Statistical analysis
Baseline characteristics of non-surviving patients were summarised as frequencies and percentages for categorical variables and as means and standard deviations for continuous variables. Comparisons by cause of death (cardiovascular death versus non-cardiovascular death, and sudden death versus non-sudden death) were performed using chi square/Fisher’s exact test for categorical variables and Student t-test for continuous variables. Mantel-Haenszel chi-square was used for test of trend over time. Univariable and multivariable Cox proportional hazards models were developed to evaluate the relations between baseline characteristics and the occurrence of cardiovascular death, and separately for the occurrence of sudden death. Kaplan-Meier life-table event rates are presented graphically.
To minimise Type I errors, it was pre-specified by the study protocol that a p-value of less than 0.01 would be considered as showing evidence of differences in secondary analysis.
SAS version 9.1.3 (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
Causes of death
Of 2,201 study patients, 1,101 had been randomly assigned to routine PCI plus optimal medical therapy, and 1,100 to optimal medical therapy alone. Death occurred in 184 patients. Analysis was based on 174 cases with available cause of death information (Table 1).
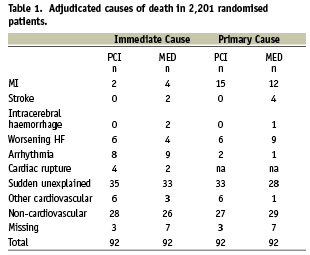
Of these, 42 patients had reached a component of the primary endpoint prior to death, including 24 patients with class IV heart failure, 11 patients with MI, and seven patients with both heart failure and MI. Patients who had a recurrent MI prior to death were more likely to die of recurrent MI (immediate death in 27.3%, primary cause of death in 40.9%). Frequent cause of death in patients with classes III or IV heart failure was heart failure class IV (immediate death in 25%, primary cause of death in 41.7%). Sudden unexplained deaths occurred mainly in patients who had not had a previous event (40% of patients, compared with 26% of patients who had had a previous event). There were no statistically significant differences in the distribution of causes of death by treatment group (Table 1). During follow-up, 49 patients received implantable automated defibrillators (ICDs, 28 PCI assigned, 21 MED assigned patients). Primary cause of death in eight patients with AICD was worsening heart failure in four patients (two MED and two PCI assigned), sudden death in two (one after failed PCI, and one assigned to MED), and non-cardiovascular in two patients who had been assigned to PCI.
PCI-related death occurred in four PCI group patients and two MED patients undergoing PCI, with two patients dying from cardiac rupture, two from arrhythmia, and two patients dying from stent thromboses at six and eight days from implantation.
Baseline characteristics of patients by survival status
While baseline characteristics of the patients in the two treatment groups were similar except for a higher prevalence of diabetes in the group assigned to MED, baseline characteristics of patients suffering death versus survivors differed significantly with regard to age (62.7 [30–88] versus 58.2 [24–89] years, p<0.0001), previous PCI (9.8 versus 4.3%, p<0. 001), diabetes (31.5% versus 19.6%, p=0.0001), history of angina (34.8% vs. 21.4%, p<0.0001), history of hypertension (60.3% vs. 47.6%, p<0.001), history of cerebrovascular disease (8.7% vs. 3.3%, p<0.001), history of chronic heart failure (CHF, 10.9% vs. 1.6%, p<0.0001), pre-existing NYHA functional class (Classes >I in 33.7% compared with 19.7%, p<0.0001) and Killip classes (Classes II-IV in 37.7% compared with 17.3%, p<0.0001). Glomerular filtration rate at baseline was <60 ml/min/1.73 m2 in 28.7% of patients eventually suffering death, compared with 13.5% of patients surviving (p<0.0001). Time-to-randomisation from index MI (9.8 [6.6 days]) versus 11.1 (7.8 days), (medians ±SD, P=0.03) was shorter in patients who died.
Anterior and lateral ST elevations were more common in non-survivors (anterior, p=0.01, lateral, p<0.01). These electrocardiographic findings were in accord with the angiographic findings of more frequent LAD occlusion in non-survivors (45.6% as compared with 35.2% in survivors, p=0.003). Collateral grades and TIMI flow in the IRA were similar in non-survivors and survivors. Multivessel disease was more frequent in non-survivors (25.5% versus 16.6%, p=0.002). LVEF assessed by echo (29%), radionuclide ventriculography (3.4%) or by left ventricular angiography (67.5%) at baseline was lower in non-survivors (40.8%±11.3%) compared with survivors (46.9%±10.6, p<0.0001).
There were higher rates of use of diuretics (37% vs. 15%, p<0.0001) at hospital discharge, digitalis (11% vs. 2% p<0.0001) and insulin (13% vs. 6% p<0.0001) in patients later suffering death. By contrast, use of lipid-lowering medication was more prevalent in survivors (dead, 71.2%, versus survivors, 82.2%). Use rates of beta blockers, angiotensin converting enzyme inhibitors and aldosterone antagonists were similar in patients who survived and those who did not, as were other medication use rates (data not shown).
Baseline characteristics of patients suffering non-cardiovascular compared with cardiovascular death (Table 2)
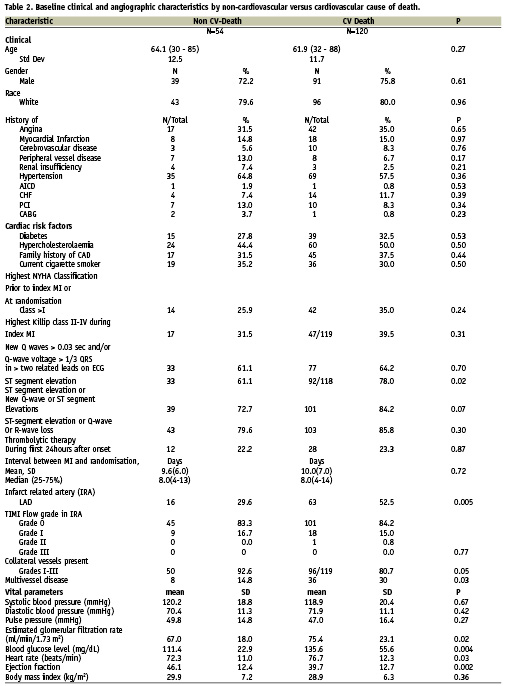
When patients were stratified into those suffering cardiovascular versus non-cardiovascular death, baseline characteristics did not differ between groups, with the exception of ST elevation during the index MI, which was more frequent in patients suffering cardiovascular death. Heart rate (77 versus 72 beats per minute, and blood glucose (136 versus 111 mg/dl) were also higher among those who died of cardiac causes. There were no differences in cardiovascular, antiplatelet and anti-diabetic medication use rates among non-survivors grouped by cause of death with the exception of digitalis, which was used far less in patients suffering sudden cardiac death in comparison with patients suffering non-sudden cardiac death (4.4% versus 14.4%).
There were no differences in the use of thrombolytic agents during the first 24 hours after onset of index MI (22.2% versus 23.3%, p=0.87) in patients suffering non-cardiovascular death versus patients suffering cardiovascular death (Table 2). By contrast, glycoprotein IIb/IIIa antagonists were employed more frequently in patients later suffering a cardiovascular death (p=0.03). However, bleeding was in no instance reported as a cause of death.
At angiography, occlusion of the LAD was observed more often in patients suffering cardiovascular death (52.5% vs. 29.6% p=0.005, Table 2). Multivessel disease was more frequent in patients suffering cardiovascular death (30.0% vs. 14.8%, p=0.03, Table 2). A left ventricular angiogram demonstrated lower ejection fractions in patients suffering cardiovascular death (39.7 vs. 46.1, p=0.002, Table 2).
Baseline characteristics stratified by immediate causes of death
The variables shown in Table 2 were examined for their relationship to immediate cause of death. Patients dying from heart failure had highest Killip classes during the index MI, and the majority of these patients were in NYHA classes >I at randomisation. Patients suffering “other immediate cardiovascular death” were diabetic in roughly two thirds of cases. No statistical tests were performed because of the small size of subsets.
Timelines of causes of death
Sudden death and other cardiovascular death occurred mainly within the first six months after randomisation (p<0.001, Figure 1). Arrhythmia accounted for 10% of deaths each year over five years observation. MI and stroke occurred mainly over the initial two years of observation. In year five the leading cause of death was non-cardiovascular (7/14). Figure 2 demonstrates Kaplan-Meier event curves by treatment group for cardiovascular death and sudden death. No differences were observed according to assigned treatment.
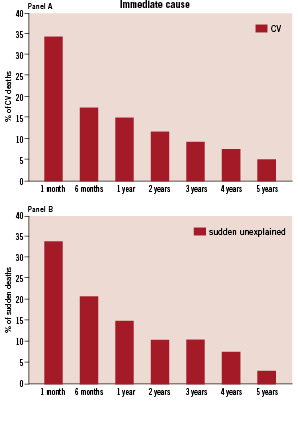
Figure 1. Timeline of occurrence of immediate cardiovascular (Panel A) and sudden death (Panel B). Panel A demonstrates the percent distribution of CV death for the 120 CV deaths. Panel B shows percent distribution of sudden death for the 68 sudden deaths
Predictors of all-cause mortality and cardiovascular death
In multivariable analysis, independent predictors of death were Killip class>1 during index MI {HR 1.75, p<0.001}, history of cerebrovascular disease {HR 2.28, p=0.002}, angina {HR 1.57, p=0.005}, history of heart failure {HR 2.11, p=0.004}, EF {HR 1.51 per 10% decrease, p<0.001}, days from MI to randomisation {HR 1.03 per day decrease, p=0.008}, and reduced GFR {HR 1.18, per 10 mL/min/1.73 m2, p<0.001}.
Utilising Cox multivariable analysis, baseline variables independently associated with cardiovascular versus non-cardiovascular death were lower ejection fraction (p<0.01) and higher glomerular filtration rate (p<0.05). Variables independently associated with sudden cardiac death versus non-sudden death were lower ejection fraction (p<0.001) and younger age (p=0.001).
Cardiac rupture
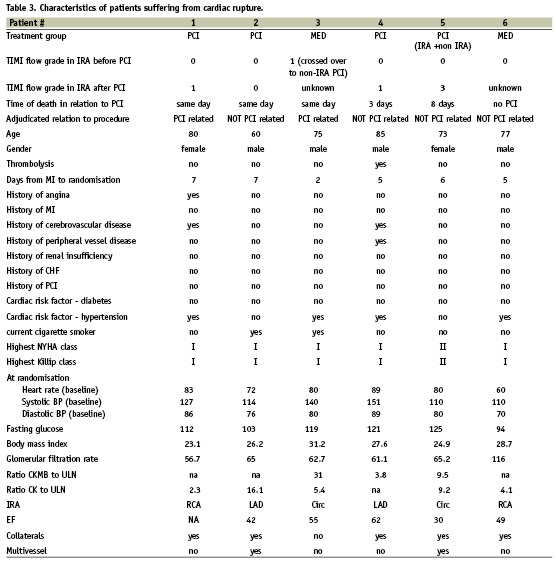
Detailed characteristics of patients dying from cardiac rupture are shown in Table 3. Rupture of the free ventricular wall as an immediate cause of death occurred in four patients of the PCI group (on the same day in two patients, and on the 3rd and 8th day in two patients), and in two patients of the MED group. One of the latter had crossed over to PCI and suffered a PCI-related cardiac rupture. Thus, 83.3% of free wall rupture occurred within eight days of PCI (99% CI by exact binominal Clopper-Pearson test, 25.4% to 99.9%, p=0.22). Among these patients, one died of rupture after non-IRA PCI, and another after IRA and non-IRA PCI (patients #3 and 5, Table 3). Prior fibrinolytic therapy had been given to only 1/6 patients dying of cardiac rupture. Of the four patients with a reported final TIMI flow grade, TIMI 3 flow was achieved in only one.
Discussion
OAT challenged the late “open artery hypothesis” by demonstrating no reduction in death, reinfarction or heart failure during 5-year follow-up. These results confirm previous small studies17, but came as some surprise because evidence for the superiority of primary PCI over medical therapy in the treatment of acute MI within 6-12 hours after onset of symptoms is overwhelming18. Because of the observed trend toward an excess risk of reinfarction in the PCI group8, and evidence that suggests heart failure and sudden death may be reduced with PCI19, a detailed descriptive analysis of causes of death was performed.
Immediate and primary causes of death were equally distributed between the assigned treatment groups, affecting 92 patients each. Patients suffering death were generally older with comorbid conditions, e.g., diabetes and hypertension, and had lower LVEF as well as a higher likelihood of multivessel disease. Differences in medication use likely reflect poorer cardiovascular health status in the individuals suffering later death. Lower LVEF was a predictor of sudden and other cardiovascular death, while impaired kidney function predicted non-cardiovascular death. Older age predicted non-sudden death. If another component of the primary endpoint occurred prior to censoring for death, this event was likely to represent the cause of death. Predictors of death were overlapping with independent predictors of the composite outcome20.
Contrary to hypothesis, when deaths were stratified by treatment assignment, no differences in cause or timing of death were evident. According to MMCC review of deaths, before the uniform Academic Research Consortium criteria21, only two stent thromboses occurred as cause of death, suggesting that this complication did not play a predominant role in the outcomes of OAT.
Few contemporary comparisons are available with early survivors of MI looking at long-term causes of death. In a landmark study describing the benefit of primary PCI over thrombolysis in acute MI who survived to 30 days, 5-year cumulative causes of death in the entire group were cardiac in 38 patients (9.6%), with a single case of cardiac rupture (0.2%), CHF in 14 cases (3.5%), and sudden death in 23 cases (5.8%)22. In a more recent study23, the follow-up after AMI demonstrated a total of 158 cases of cardiac death in 1,009 patients (15.6%), with 68 patients dying within the first 30 days after AMI, seven of cardiac rupture (0.6%). The one-year cardiovascular death rate in OAT was 3.8%, with a rate of 0.9% mortality per year over the next four years. These data suggest a reduction in cardiac mortality with contemporary treatments. The observation in OAT that cardiovascular mortality decreased during the first year after MI and stabilised thereafter is in accord with published literature23,24.
Cardiac rupture
Causes of death in patients who have survived at least 24 hours are skewed towards fewer acute arrhythmias and cardiogenic shock because OAT patients are, by definition, survivors of the early fatal arrhythmia and shock risk period. As a consequence, the relative proportion of cardiac rupture is higher in late presenters in general, and specifically in the OAT population, than would be expected in an overall STEMI population. Still, the frequency of free wall rupture (FWR) in the OAT PCI group is low (0,36% (4/1101) by “intention to treat” analysis, and 0,45% (5/1101) by “on treatment” analysis), and as such much lower than the reported risk of mechanical complications of MI treated with primary PCI (1.8%25 and 2.2%26), or after combined primary PCI and thrombolysis (2.7 with optimal and 4.9% with failed PCI27). However, among the 41,021 patients in the Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial, ventricular septal rupture was confirmed by a retrospective review in 84 patients (0.2 percent)28.
Although not statistically significant, it is interesting to note that five of six cases of FWR occurred in patients undergoing PCI (Table 3). Rupture of the free wall of the left ventricle is the second most common cause of death from acute MI in the coronary care unit, pump failure being the most common. Risk factors for rupture include older age, female sex, first infarction, moderate to large infarct size, an increased time delay from symptoms to treatment, hypertension, and absence of ventricular hypertrophy29,30. These characteristics apply to patients suffering rupture in OAT, with the exception that these were not more likely to be female (Table 3). Patients suffering rupture in OAT had documented collaterals to the IRA suggesting more long-standing ischaemia. The typical presentation of rupture is sudden cardiovascular collapse and death, but up to 30 percent of patients have a sub-acute form of rupture that may allow time for diagnosis and surgical intervention, with a greater possibility of survival31-34. Four of the cases of FWR in OAT were adjudicated as not PCI-related but did occur within eight days of PCI. This is in keeping with current understanding of the timing of myocardial rupture, which has a bimodal time distribution, with approximately two-thirds occurring during the week after infarction, and one third within the first 24 hours30,35. In OAT, reperfusion timelines were covering a wide time window from >24 hours after the infarction to more than 200 hours later. It remains unknown when in the process if infarction the myocardium is most sensitive to rupture. Within a certain time window, successful PCI may decrease the area at risk and thus limit infarct expansion36, however at the cost of an increased risk of intramyocardial haemorrhage. Fibrinolytic drugs are clearly associated with an increased risk of FWR29. The take-home message is that cardiac rupture has become a rare complication of contemporary myocardial infarction treatments, but may be precipitated by late PCI within days three and eight after the infarction.
Limitations
The number of patients suffering cardiac rupture is small, hence information and statistical analyses are limited.
We do not have information on the number of deaths in the initial 90 days after stopping clopidogrel37.
Of 68 patients with sudden cardiac death, 20 may have met current criteria for ICD >30 days post MI, i.e., baseline EF<30% or NYHA Class >1 and EF < 35%38. In fact, seven of these patients suffered sudden cardiac death within 30 days of qualifying MI. Taken together, this calculation would translate into 13 deaths that may have been avoided in the current era. This figure marks an upper bound because some patients will have improved their EF by 30 days.
Although an independent MMCC adjudicated causes of death, attribution of the exact cause can be problematic in complex cases.
Conclusion
OAT provides insight into the causes of death in stable patients with persistent total occlusion of the IRA post MI, and the potential impact of delayed PCI. PCI of the culprit vessel does not affect the rate, cause or timing of death days to weeks after MI. The observation that the majority of cardiac ruptures occurred in patients undergoing PCI deserves further investigation.
Acknowledgements
The authors would like to thank Dr. Harmony R. Reynolds for her editorial input on the manuscript, and Zubin Dastur and Emily Levy for their assistance in the preparation of the manuscript.

